










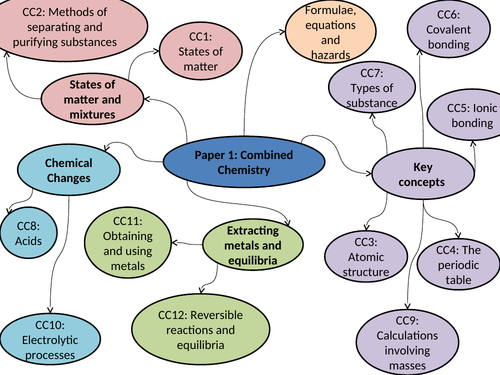










AQA GCSE 9-1 CHEMISTRY UNIT 3.1 Chemical measurements, conservation of mass, equations (no moles)
4.3.1 Chemical measurements, conservation of mass and the quantitative
interpretation of chemical equations
4.3.1.1 Conservation of mass and balanced chemical equations
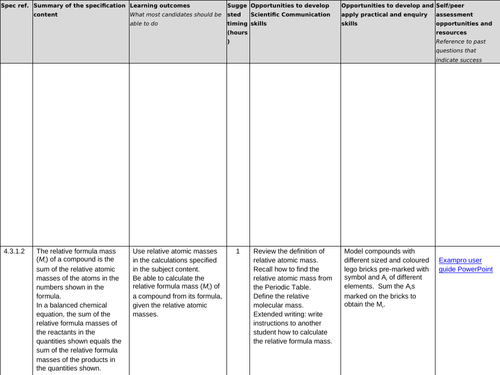
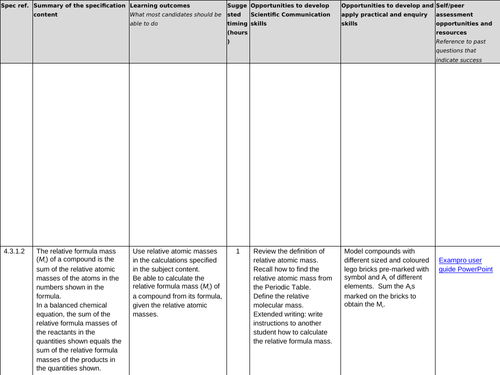
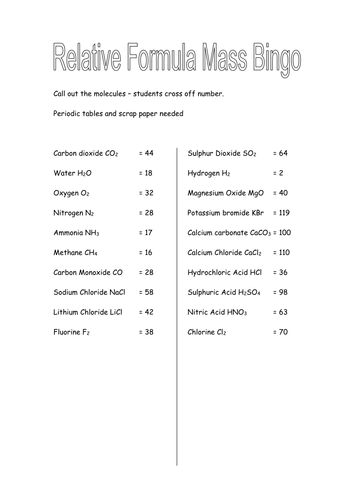
4.3.1.2 Relative formula mass
4.3.1.3 Mass changes when a reactant or product is a gas
4.3.1.4 Chemical measurements
Content split over 7 lessons (lessons in our school are 40 minutes so can condense material for longer lessons if required)
All exam questions have been removed for copyright purposes
All extension questions available on each slide
Answers all underneath each slide
Support also available where necessary
AfL sections and mini quizzes
Reducing the need for photocopying
Homework
Homework can also be used as extension sheets in lessons - or for higher ability students
EXTRA LESSONS INCLUDE (as we teach it anyway in our school to help with A-level) Molecular to empirical conversion, empirical to molecular conversion, empircial formula practical and balancing equations with skittles.
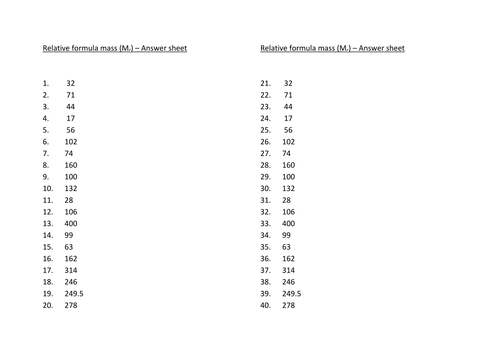
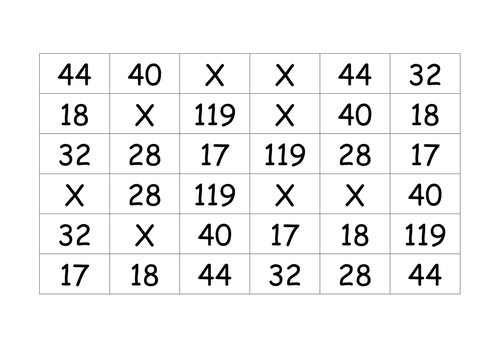
Lesson 1: Relative formula mass
To know the term relative atomic mass (Ar)
To be able to count atoms
To be able to use the formulae of a substance to calculate its mass (Mr)
Lesson 2: Molecular formula
To know the terms molecular and empirical formula
To be able to deduce the molecular formula of a compound from its empirical formula and its relative molecular mass
To consolidate learning
Lesson 3: Empirical formula
To be able to calculate the formulae of simple compounds from reacting masses and understand that these are empirical formulae
To consolidate learning with questions
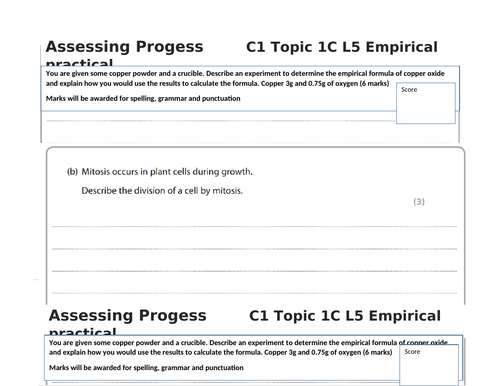
Lesson 4: Empirical formula practical
To know how to carry out a practical to determine the empirical formula of a compound
To be able to calculate the empirical formula of a simple compound such as magnesium oxide
To consolidate learning with questions (BS booklet)
Lesson 5: Conservation of mass - enclosed system
To understand the terms closed and non-enclosed systems
To investigate what happens to the reactants and products in a closed system
To understand what the law of conservation of mass is
Lesson 6: Conservation of mass - non enclosed system
To be able to apply the law of conservation of mass to a non-enclosed system
To be able to draw the particle arrangements of reactants and products
To consolidate learning with questions
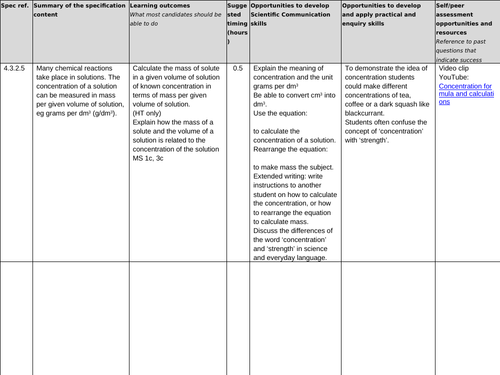
Lesson 7: Concentration g/dm3
To be able to define the term ‘concentration’ (H)
To be able to convert between cm3 and dm3
To understand how to calculate the concentration of solutions in g dm-3 or g/dm3
EXTRA:
Lesson 8a
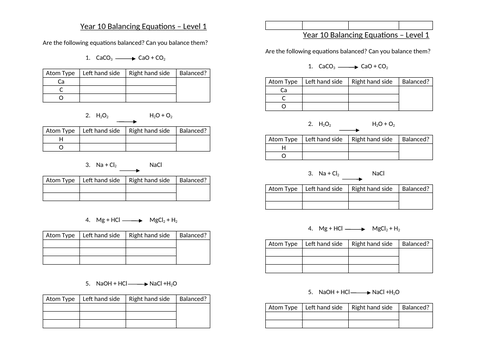
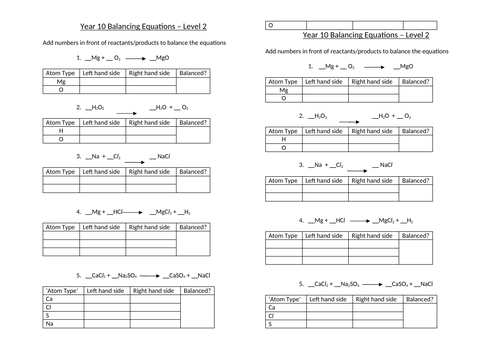
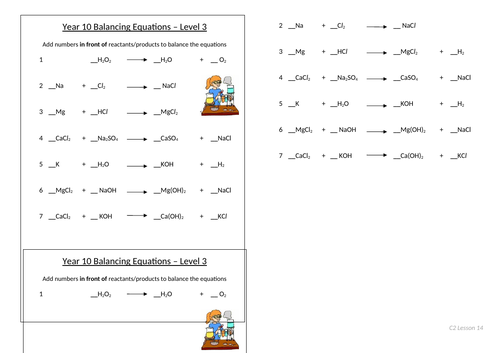
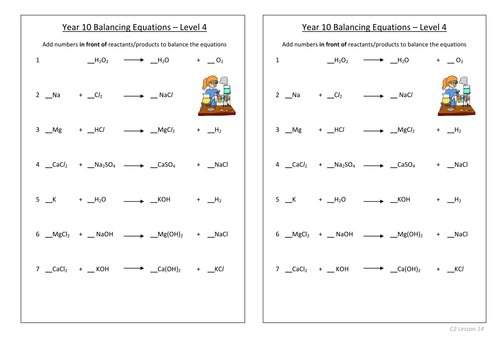
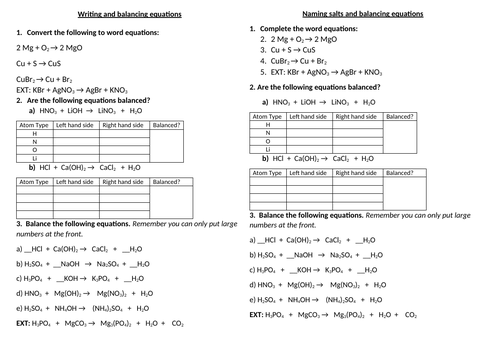
Balancing equations
To be able to count atoms in equations
To be able to understand how to balance equations
To consolidate learning with questions
Lesson 8b
…with skittles
To recall the term relative atomic mass (Ar) and relative formula mass (Mr)
To be able to count atoms in equations
To be able to understand how to balance equations
Get this resource as part of a bundle and save up to 26%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.