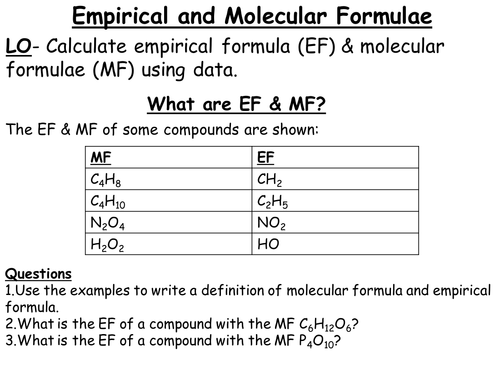
1) The PowerPoint initially introduces the difference between empirical and molecular formula.
2) The teacher then works through some examples of calculating empirical and molecular formula using a table as a guide.
3) Students carry out calculations on their own/in groups using the template.
4) The teacher introduces calculating empirical formula of hydrated compounds. Then the students attempt a question.
5) Past paper questions and a mark scheme are attached for students to subsequently attempt.
6) A set of instructions is also attached for students to deduce the empirical formula of magnesium oxide using their own experimental evidence.
2) The teacher then works through some examples of calculating empirical and molecular formula using a table as a guide.
3) Students carry out calculations on their own/in groups using the template.
4) The teacher introduces calculating empirical formula of hydrated compounds. Then the students attempt a question.
5) Past paper questions and a mark scheme are attached for students to subsequently attempt.
6) A set of instructions is also attached for students to deduce the empirical formula of magnesium oxide using their own experimental evidence.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00