
This lesson describes an ion as an atom with a positive or negative charge, and explains how cations and anions are formed in ionic compounds. The lesson PowerPoint and accompanying worksheet have been designed to cover points 1.22 - 1.24 of the Edexcel GCSE Chemistry specification and also covers the same points on the Combined Science course.
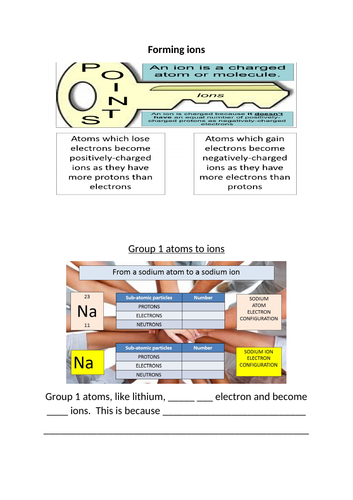
The first part of the lesson focuses on atoms and specifically on getting students to recall that they contain the same number of protons and electrons and this is why they have no overall charge. By ensuring that they are confident with this fact, they will be able to understand why ions have a charge. Students will learn that ions have full outer shells of electrons and this change in the number of this sub-atomic particle leads to the charge. They are shown examples with aluminium and oxide ions and then are challenged to apply this new-found knowledge to a task where they have to explain how group 1, 2, 5 and 7 atoms become ions. The final part of the lesson looks at how ion knowledge can be assessed in a question as they have to recognise the electron configuration of one and describe how many sub-atomic particles are found in different examples. There are regular progress checks throughout the lesson to allow the students to check on their understanding.
This lesson has been written for GCSE students but could be used with higher ability KS3 students who are looking to extend their knowledge past basic atomic structure
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.