





Reactivity 1.2 - Energy cycles in reactions
Reactivity 1.2.1 - Bond-breaking absorbs and bond-forming releases energy.
Reactivity 1.2.2 - Hess’s law states that the enthalpy change for a reaction is independent of the pathway between the initial and final states.
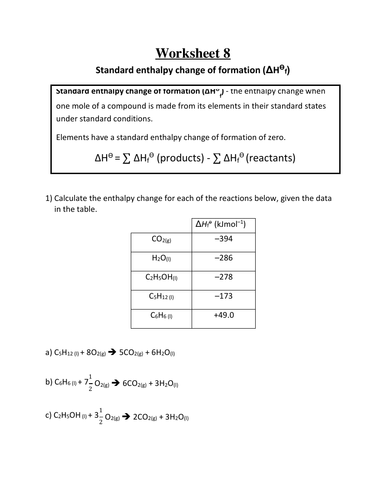
Reactivity 1.2.3 - Standard enthalpy changes of combustion, ΔHc ⦵, and formation, ΔHf ⦵, data are used in thermodynamic calculations.
Reactivity 1.2.4 - An application of Hess’s law uses enthalpy of formation data or enthalpy of combustion data to calculate the enthalpy change of a reaction.
Reactivity 1.2.5—A Born–Haber cycle is an application of Hess’s law, used to show energy changes in the formation of an ionic compound.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
