

A structured KS5 lesson (Part 2 of 2) including starter activity, AfL work tasks and practice questions with answers on Group 2 Compounds
By the end of this lesson KS5 students should be able to:
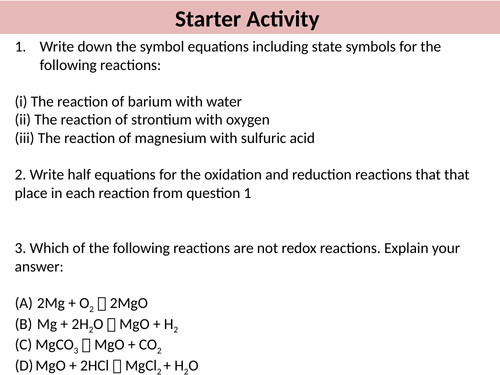
- To know the reaction between group 2 metal oxides and water
- To state the trend in solubility and alkalinity of group 2 metal hydroxides
- To describe the uses of some group 2 compounds including their equations
The teacher will be able to check students have met these learning objectives through mini AfL tasks for students to complete
Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons, including using your own lesson PowerPoints, is a fundamental skill of a qualified/unqualified teacher that will be assessed during the scenarios outlined above
Get this resource as part of a bundle and save up to 20%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
OCR Chemistry: Group 2 Elements & Compounds
2 Lesson bundle covering the OCR Periodic Table Chapter on group 2 elements and compounds **Lesson 1: Group 2 Elements** By the end of this lesson. Students should be able: 1. To know group 2 elements lose their outer shell s2 electrons to form +2 ions 2. To state and explain the trend in first and second ionisation energies of group 2 elements and how this links to their relative reactivities with oxygen, water and dilute acids 3. To construct half equations of redox reactions of group 2 elements with oxygen, water and dilute acids and to identify what species have been oxidised and reduced using oxidation numbers **Lesson 2: Group 2 Compounds.** By the end of this lesson students should be able: 1. To know the reaction between group 2 metal oxides and water 2. To state the trend in solubility and alkalinity of group 2 metal hydroxides 3. To describe the uses of some group 2 compounds including their equations The teacher will be able to check students have met these learning objectives through mini AfL tasks for students to complete ***Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons, including using your own lesson PowerPoints, is a fundamental skill of a qualified/unqualified teacher that will be assessed during the scenarios outlined above***
The Periodic Table (OCR)
9 Full Lesson Bundle covering **Module 3.1 - The Periodic Table** from OCR A Level Chemistry A specification. Please review the learning objectives below Lesson 1: The Structure of The Periodic Table 1. To know how the periodic table is arranged 2. To describe the periodic trend in electron configurations across periods 2 and 3 3. To classify elements into s, p and d blocks Lesson 2: AS Chemistry: Ionisation Energy (Part 1) 1. To define the term ‘first ionisation energy’ and successive ionisation energies 2. To describe the factors affecting ionisation energy 3. To explain the trend in successive ionisation energies of an element Lesson 3: AS Chemistry: Ionisation Energy (Part 2) 1. To explain the trend in first ionisation energies down a group 2. To explain the trend in first ionisation energies across period 2 3. To explain the trend in first ionisation energies across period 3 Lesson 4: Periodicity: Melting Points 1. To describe the trend in structure from giant metallic to giant covalent to simple molecular lattice 2. To explain the variation in melting points across period 2 & 3 in terms of structure and bonding Lesson 5: AS Chemistry: Group 2 Elements 1. To know group 2 elements lose their outer shell s2 electrons to form +2 ions 2. To state and explain the trend in first and second ionisation energies of group 2 elements and how this links to their relative reactivities with oxygen, water and dilute acids 3. To onstruct half equations of redox reactions of group 2 elements with oxygen, water and dilute acids and to identify what species have been oxidised and reduced using oxidation numbers Lesson 6: AS Chemistry: Group 2 Compounds 1. To know the reaction between group 2 metal oxides and water 2. To state the trend in solubility and alkalinity of group 2 metal hydroxides 3. To describe the uses of some group 2 compounds including their equations Lesson 7: The Halogens: Properties & Reactivity 1. To describe and explain the trend in boiling points of the halogens in terms of induced dipole-dipole interactions (London Forces) 2. To describe and explain the trend in reactivity of the halogens illustrated by their displacement reaction with other halide ions 3. To construct full and ionic equations of halogen-halide displacement reactions and to predict the colour changes of these reactions in aqueous and organic solutions Lesson 8: Disproportionation & The Uses of Chlorine 1. To explain the term disproportionation 2. To explain how the reaction of chlorine with water or cold dilute sodium hydroxide are examples of disproportionation reactions 3. To evaluate the uses of chlorine (How Science Works) Lesson 9: Qualitative Analysis 1. To carry out test tube reactions and record observations to determine the presence of the following anions : CO32- SO42- , Cl-, Br-, and I- 2. To carry out test tube reactions and record observations to determine the presence of the following cations: NH4+, Fe2+, Fe3+, Mn2+ and Cu2+ 3. To construct ionic equations to explain the qualitative analysis tests of cations and anions ***Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons including using your own lesson PowerPoints is a fundamental skill of a qualified/unqualified teacher that will be reviewed during these scenarios outlined above***
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.