



This lesson covers an introduction to atomic theory.
In includes a Power Point lesson covered the topics outline below. It also includes a revision or homework page (with answer sheet). A ‘Hot Seat Quiz’ that includes the key terms covered in the lesson and a printable of the key figures in Atomic Theory.
The Power Point lesson covers the key terms:
• Atomic theory
• Atoms
• Atomic structure
• Protons
• Neutrons
• Electrons
• Atomic Mass Unit
This is followed by a section covering describing atoms:
• Electron configuration
• Mass Number (Nucleon number)
• Atomic number (proton number)
The last section covers briefly
• Isotopes
• Relative Mass Unit
• Relative Formula Mass
Designed to partially cover section C3 of the iGCSE unit 0653 combined science CIE course as outlined on the Cambridge Specification here:
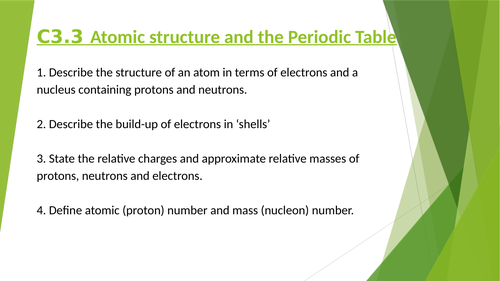
3.3 Atomic structure and the Periodic Table
1 Describe the structure of an atom in terms of electrons and a nucleus containing protons and neutrons.
2 Describe the build-up of electrons in ‘shells’ and understand the significance of the noble gas electronic structures and of valency electrons (reference to noble gases not included - this will be in periodic table lesson)
3 State the relative charges and approximate relative masses of protons, neutrons and electrons.
4 Define atomic (proton) number and mass (nucleon) number.
5 Use proton number and the simple structure of atoms to explain the basis of the Periodic Table ((Not yet included in this section)
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.