


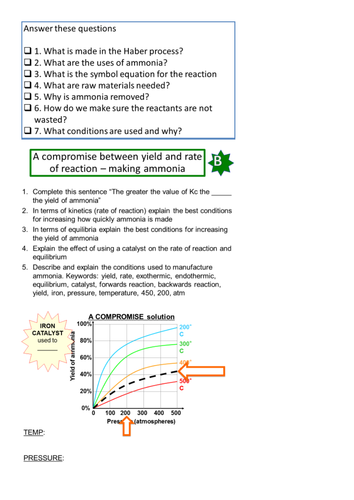
This lesson forms part of an AS chemistry equilibrium scheme of work and follows on from two lessons on equilibrium reactions and writing expressions for Kc. The lesson starts with a recap of Kc. Students then learn how to work out the units for Kc. Please note that from experience I have found that weaker students (grade C downwards) struggle with this so please take a lot of time to check that students feel comfortable and confident. A GSCE indices questions worksheet has been provided to support weaker students. The lesson then moves on to explaining the compromise conditions used to make ammonia in the Haber process. I show the Daniel D Dulek TED talk video here. It is absolutely excellent and stretches the students. Video questions are provided. The lesson concludes with students calculating Kc. The video is YouTube embedded so please download this video before the lesson as many schools do not allow staff access to YouTube from a school computer. Please rate this resource and leave feedback.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00