
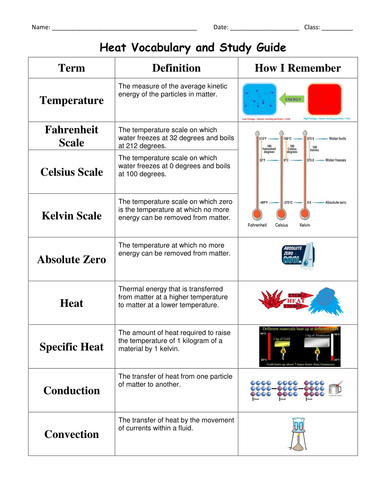

This is the study guide I use with my students as they prepare for their unit test. The vocabulary includes a space for students to write the definitions as well as a space for them to draw a picture, write an example, or anything else that helps them remember. This is followed by heat review questions. Full answer key with work solved out and explanations included. Study guide including vocabulary is 7 pages long. Also includes 3 powerpoint slides including 2 days' activators and a slide of the daily objectives that accompany this unit.
Vocabulary review suggestion - print out single sided. Put numbers on the back to match up the word and the definition. Cut out and play matching!! (Numbers allow students to check their matches).
Aligned to the following MA state standards for High School Introductory Physics:
3.1 Explain how heat energy is transferred by convection, conduction, and radiation.
3.2 Explain how heat energy will move from a higher temperature to a lower temperature until equilibrium is reached.
3.3 Describe the relationship between average molecular kinetic energy and temperature. Recognize that energy is absorbed when a substance changes from a solid to a liquid to a gas, and that energy is released when a substance changes from a gas to a liquid to a solid. Explain the relationships among evaporation, condensation, cooling, and warming.
3.4 Explain the relationships among temperature changes in a substance, the amount of heat transferred, the amount (mass) of the substance, and the specific heat of the substance.
Vocabulary review suggestion - print out single sided. Put numbers on the back to match up the word and the definition. Cut out and play matching!! (Numbers allow students to check their matches).
Aligned to the following MA state standards for High School Introductory Physics:
3.1 Explain how heat energy is transferred by convection, conduction, and radiation.
3.2 Explain how heat energy will move from a higher temperature to a lower temperature until equilibrium is reached.
3.3 Describe the relationship between average molecular kinetic energy and temperature. Recognize that energy is absorbed when a substance changes from a solid to a liquid to a gas, and that energy is released when a substance changes from a gas to a liquid to a solid. Explain the relationships among evaporation, condensation, cooling, and warming.
3.4 Explain the relationships among temperature changes in a substance, the amount of heat transferred, the amount (mass) of the substance, and the specific heat of the substance.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
$3.00
