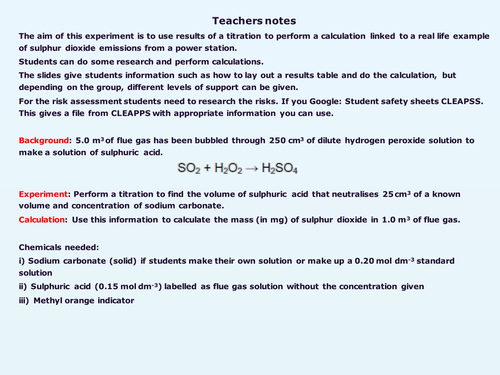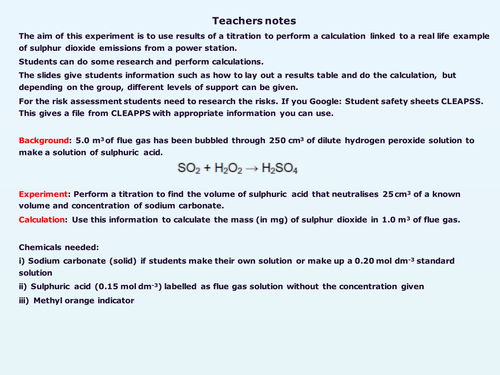
The aim of this experiment is to use results of a titration to perform a calculation linked to a real life example of measuring sulphur dioxide emissions from a power station. The scenario is that students are given a solution of sulfuric acid produced when waste gas from a power station is bubbled through hydrogen peroxide. They perform a titration the sulfuric acid against a known solution of sodium carbonate then use the results to calculate the mass of sulfur dioxide in the waste gas.
Included in the PowerPoint is an introduction to the experiment, teachers notes, results table, instructions for students, advice on performing titrations and how to do the calculations. Depending on the group, different levels of support can be given.
Included in the PowerPoint is an introduction to the experiment, teachers notes, results table, instructions for students, advice on performing titrations and how to do the calculations. Depending on the group, different levels of support can be given.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
$2.00