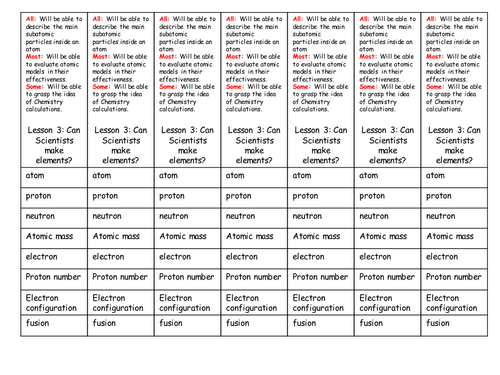
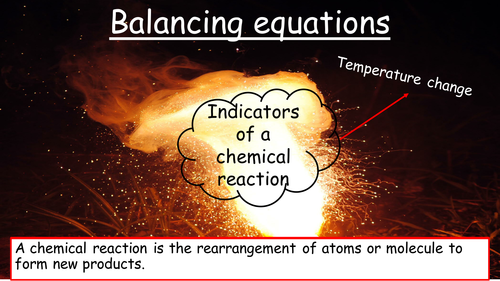
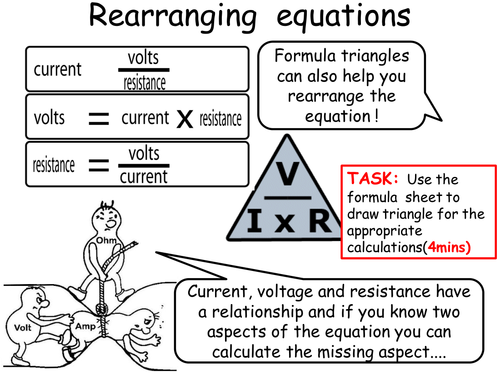
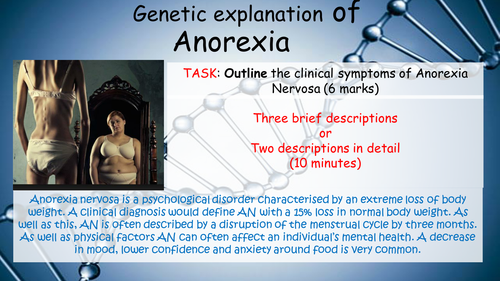
Mick Doyle's Resource Shop
Over the last five years I have found the best way to stimulate learning is through engaging lessons. Lessons which apply scientific content to unusual, topical or popular scenarios. I currently have a range of premium and free resources to look through. I will continue to upload these resources as and when I can. Feel free to review, tweet or contact me regarding these resources or for ideas on current topics you are struggling to make engaging.