





AQA A2 Level Electochemical cells (3.1.11) - Electrochemical series complete lesson package
Using the specification and books
No exam questions are included due to copy right
Including:
Homework booklets
Assessment sheets
Interactive powerpoints (rarely seen in A-level)
You will need a membership to Chemsheets - doesn’t have to be used with chemsheets
RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE
Very detailed- will not suit everyone (hidden slides are extras depending on ability of class)
3.1.11 Electrochemical cells
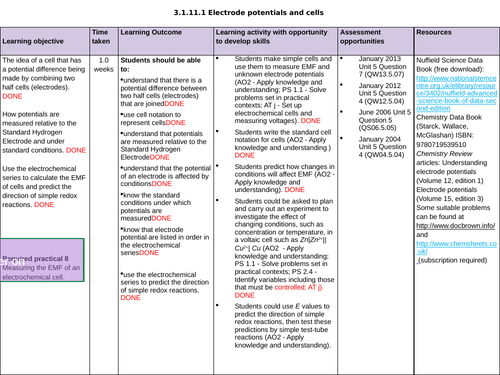
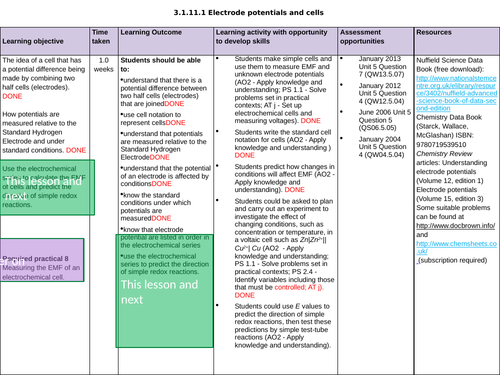
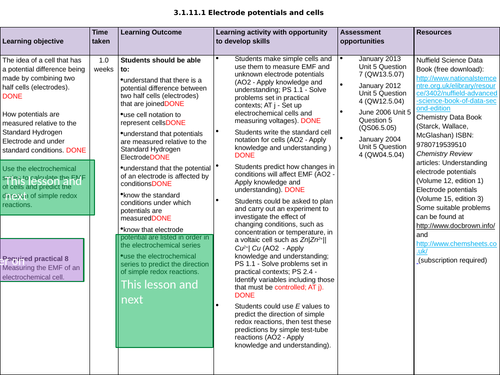
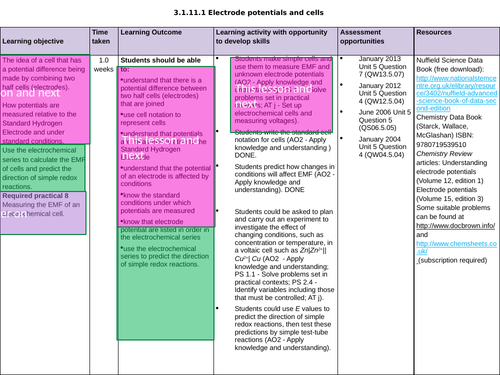
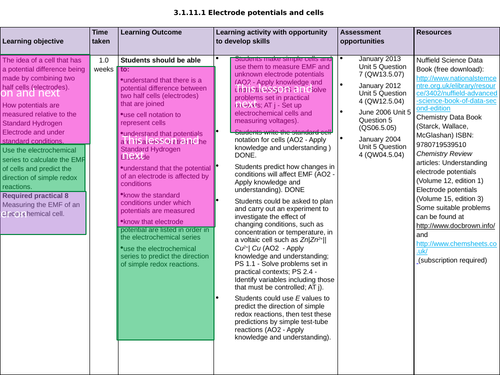
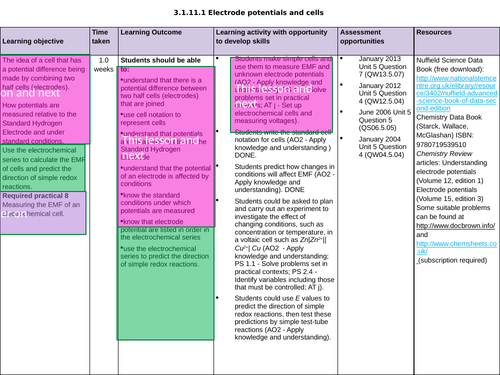
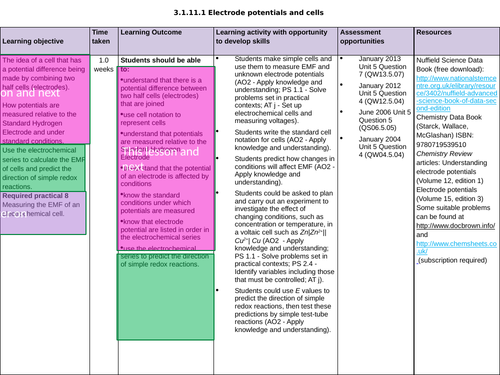
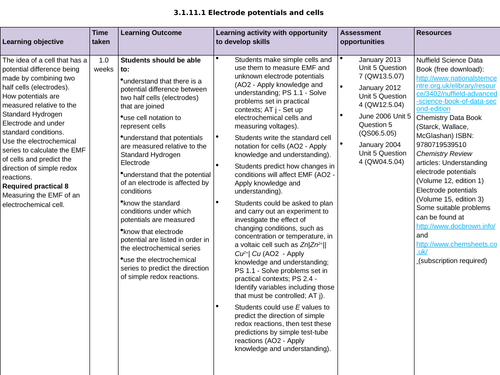
3.1.11.1 Electrode potentials and cells
3.1.11.2 Commercial applications of electrochemical cells
LESSON 1: Recap
L1- Oxidation and Reduction from AS-Level
AS LINK https://www.tes.com/teaching-resource/resource-12099917
Exam Q
Objectives:
To familiarize yourselves with the key concepts of oxidation states from AS Chemistry
To recap redox reactions
To recall the terms oxidising agent and reducing agent
L1b Exam question consolidation *
L2a Electrochemical cells PART 1
The potential of an electrode
To know the importance of the conditions when measuring the electrode potential, E (Nernst equation not needed)
To understand how cells are used to measure electrode potentials by reference to standard hydrogen electrode
L2b Electrochemical cells PART 2
The secondary standard
The Daniell cell and representing electrochemical cells
To add a sign to the cell voltage on cell diagrams
To understand what happens to the emf when changing conditions
L3 PRACTICAL
Method, questions, results (no tech sheet)
L4 PRACTICAL - varying concentration
Method, questions and results (no tech sheet)
L5 The electrochemical series
To know that standard electrode potentials are listed in an electrochemical series
To work out overall equations by using the electrochemical series
To use the electrochemical series to predict the direction of simple redox reactions
To choose a suitable RAD or OAT
L5c Exam question consolidation * LINK BELOW
L5d Redox Titrations and electrochemical cells
To understand why HCl cannot be used in certain redox titrations
To understand why some spontaneous reactions do not occur
To understand how to determine the species present in solution at the end of the reaction
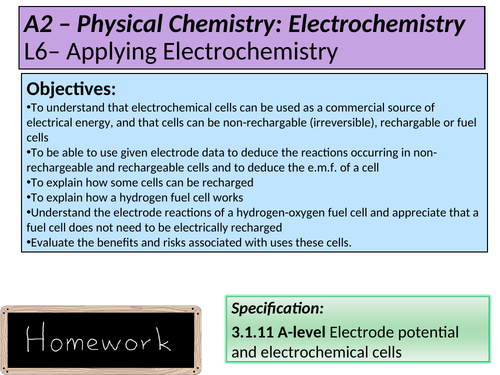
L6 + 7 Applying electrochemistry (working alongside L7b Exam question consolidation) *
To understand that electrochemical cells can be used as a commercial source of electrical energy, and that cells can be non-rechargable (irreversible), rechargable or fuel cells
To be able to use given electrode data to deduce the reactions occurring in non-rechargeable and rechargeable cells and to deduce the e.m.f. of a cell
To explain how some cells can be recharged
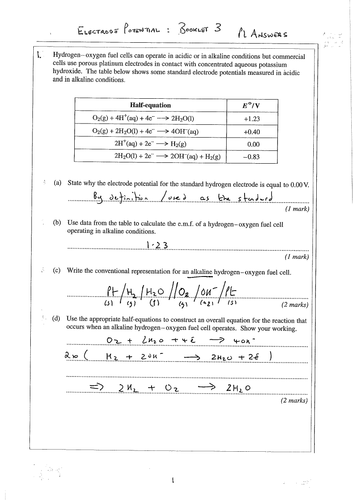
To explain how a hydrogen fuel cell works
Understand the electrode reactions of a hydrogen-oxygen fuel cell and appreciate that a fuel cell does not need to be electrically recharged
Evaluate the benefits and risks associated with uses these cells.
L8 REQUIRED PRACTICAL 8
*can’t publish ex
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.