Equilibria
Subject: Chemistry
Age range: 16+
Resource type: Assessment and revision


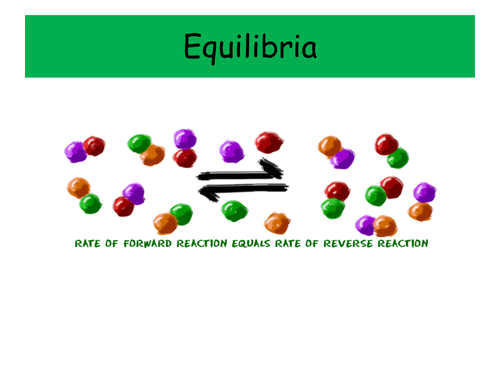
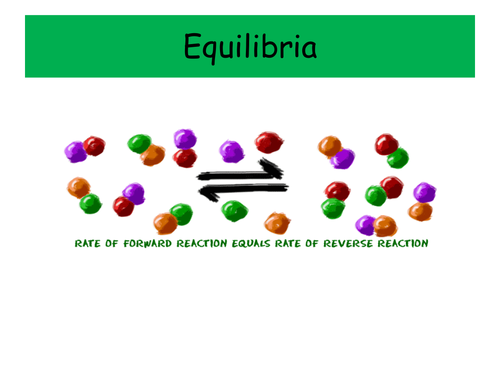
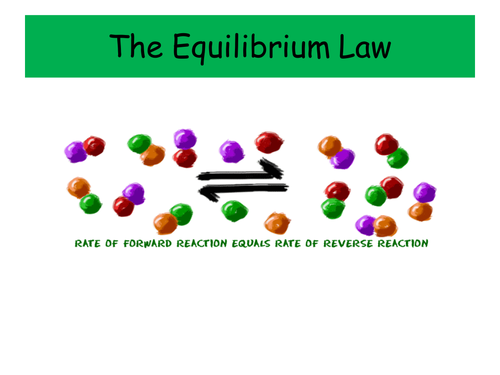
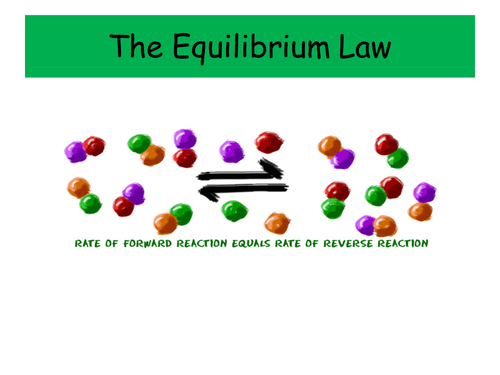
These two PowerPoints were planned as part of the IB scheme of work on Equilibria, and cover the necessary content for both the Standard and Higher Level topics. It would also be suitable for other post-16 courses.
Included are fully completed PowerPoints, student versions of the PowerPoints with sections to complete independently and some exam style questions.
Topics included are:
- The difference between reversible reactions and equilibria
- Dynamic equilibrium and the characteristic of the equilibrium state
- Physical equlibria
- What is meant by the term ´position of equilibrium´
- Le Chatelier´s Principle
- Effect of temperature on the position of equilibrium
- Effect of pressure on the position of equilibrium
- Effect of concentration on the position of equilibrium
- Effect of a catalyst on the position of equilibrium
- The General Equilibrium Law
- Calculating the equilibrium constant
- The reaction quotient
- Effect of changing reaction conditions on Kc
- Calculating the equilibrium constant from the number of moles of reaction components
- Calculating the concentrations of reaction components from the equilibrium constant
- Explaining the effect of changes in concentration on Kc
- Explaining the effect of changes in pressure on Kc
- The relationship between equilibrium and Gibbs Free Energy
- Calculating Kp
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.