states of matter and solubility - a mini course
<p>An interactive mini course</p>
<code>three states of matter in terms of the arrangement, movement and energy of the particles
phase changes in terms of the names of the interconversions and how they can be achieved
understanding how the results of experiments involving the dilution of coloured solutions and can be explained
the meaning of the terms: solvent, solute, solution, saturated solution
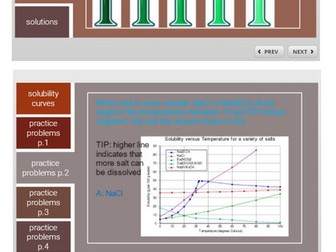
the meaning of the term: solubility in the units g per 100 g of solvent, understand how to interpret solubility curves
the factors affecting solubility
how read the solubility of a solid in water at a specific temperature
</code>
<p>How to use it: extract the zip file and open story or story_HTML5 in your browser</p>