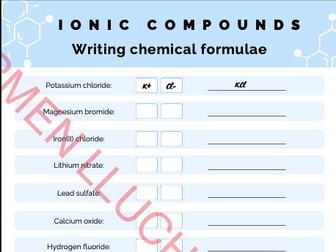
Carbon-13 & Proton NMR Card Game Activity IAL Edexcel Chemistry
<p>This resource includes an 8 page pdf, with 16 cards: clue cards, task cards and answer cards.</p>
<p>There are 4 “mistery” compounds that students have to figure out by looking at the clue cards and helping themselves with the task cards.</p>
<p>The clue cards include information about % of elements, so they must calculate the empirical formulae. Then they also have information about mass spectrometry: M+ peak + some fragments, so this is how they figure out the molecular formula. Carbon 13 and proton NMR information is included and they have to answer information about the number of peaks and splitting patterns. Additionally, there is information about chemical tests that can guide them in figuring out the compound. For example, when ethanoic acid reacts with sodium carbonate, fizzing is observed (this will aid them in the identification of ethanoic acid).</p>
<p>This has been created following the specification of International Advanced Level Edexcel Chemistry Course, but works for other specifications too.</p>
<p>For each compound, the colour code is different. The sample you can see is blue (ethyl propanoate), but there is also yellow (ethanoic acid), red (butanone) and green (3-hydroxybutanal).</p>
<p>This can be printed and laminated as if it was a card game.</p>