





AQA A2 Level Unit 5 Section 17 Thermodynamics (3.1.8) - ENtropy, enthalpy, Born-Haber cycles
Using the specification and books
No exam questions are included due to copy right
Including:
Homework booklets
Assessment sheets
Interactive powerpoints (rarely seen in A-level)
You will need a membership to Chemsheets
RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE
Unit 4 Section 17: 3.1.8 Thermodynamics
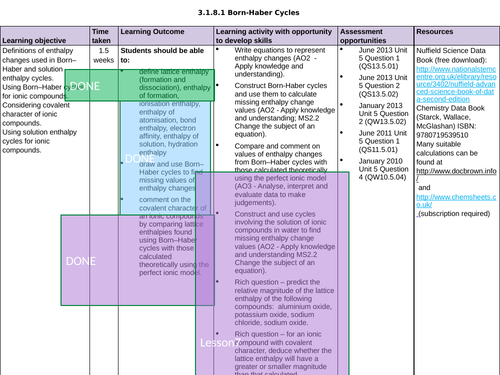
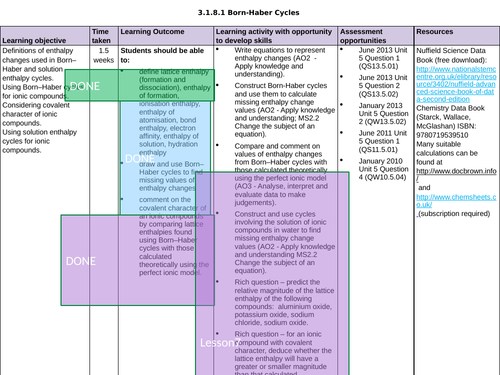
3.1.8.1 Born-Haber Cycles
3.1.8.2 Gibbs free-energy change ΔG and entropy change ΔS
AS Chemistry Link
https://www.tes.com/teaching-resource/aqa-as-level-unit-1-section-4-energetics-hess-s-law-bond-enthalpy-enthalpy-change-endo-exothermic-12093649
LESSON 1: Recap
L1- Enthalpy change and mean bond enthalpy from AS-Level
Objectives:
To have familiarised yourselves again with key concepts of enthalpy changes and Hess’s Law from AS Unit 2
To be able to define and apply the term enthalpy of formation, combustion and neutralisation
To use mean bond enthalpies to calculate approximate values of ∆H for reactions
LESSON 2: Dissolving
Objectives:
Part 1: To be able to define and apply the terms “lattice enthalpy”
Part 2: To be able to define and apply the terms “enthalpy of hydration” and “enthalpy of solution”
To calculate enthalpies of solution for ionic compounds from lattice enthalpies and enthalpies of hydration
To consolidate learning with questions
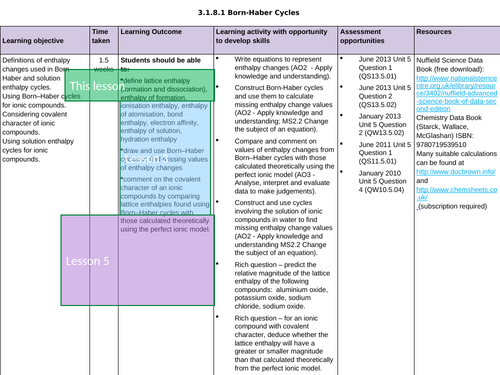
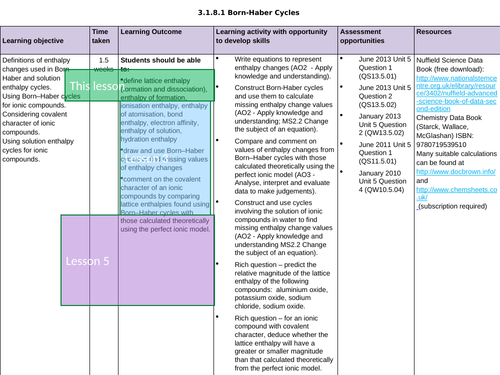
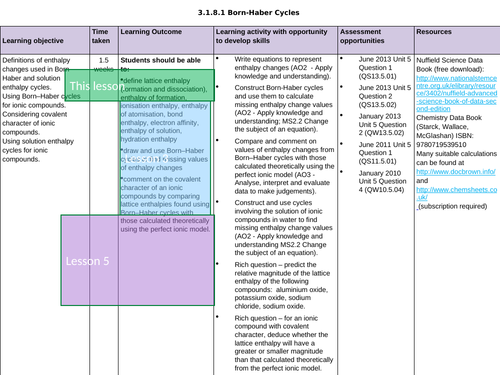
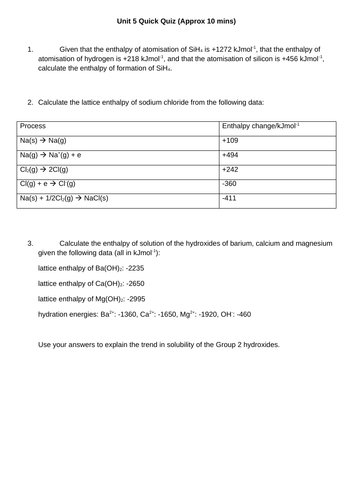
LESSON 3 + 4: Born-Haber Cycles
Objectives:
TTo be able to define and apply the terms “ionisation enthalpy”, “electron affinity” and “enthalpy of atomisation of an element and of a compound”
To draw and use Born-Haber cycles to find missing values of enthalpy changes
To consolidate learning with question
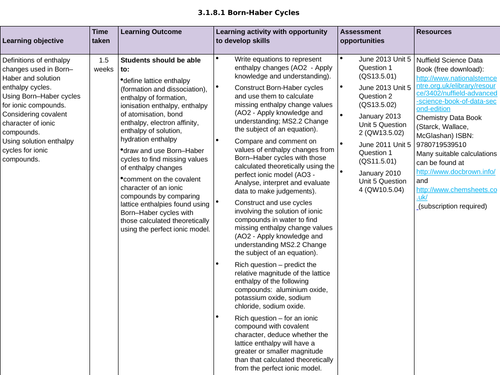
LESSON 5: Covalent Character
Objectives:
To predict enthalpy of formation of theoretical compounds
To compare lattice enthalpies from Born-Haber Cycles with those from calculations based on a perfect ionic model to provide evidence for covalent character in ionic compounds
To consolidate learning with questions
L5b Practical
L6 Entropy
To understand the term entropy
To understand the concept of increasing disorder (entropy change ∆S), illustrated by physical change, e.g., melting or evaporating and by chemical changes, e.g., dissolution, evolution of CO2 from hydrogencarbonates with acids
To be able to calculate entropy changes from absolute entropy values
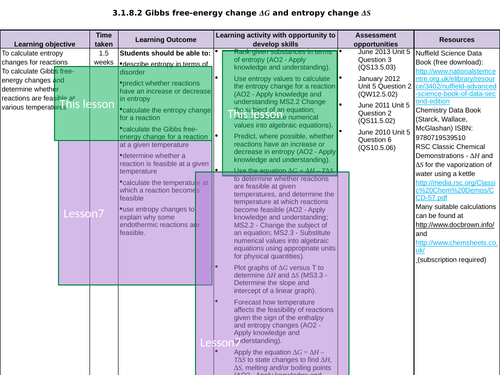
L7 + 8 Gibbs free energy and spontaneous reactions
To understand that the balance between entropy and enthalpy is given by the relationship ∆G = ∆H- ∆TS
To be able to use this relationship to determine the temperature at which a reaction is feasible
To us this equation to determine how ∆G varies with temperature and how temperature affects the feasibility of reactions given the sign of the enthalpy and entropy changes
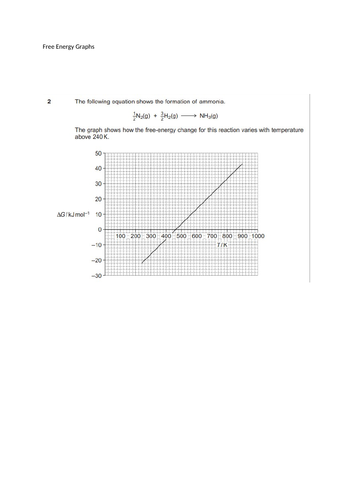
L9 Gibbs Free energy Graphs and a bit of light reading
Homework booklet with answers
Leave feedback and enjoy !!
Something went wrong, please try again later.
Thanks. Hope you had a good holiday.
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.