





AQA AS Level Unit 1 Section 6 Equilibria-Catalysts, Chemical equilibria, Le Chatelier’s principle and Kc
Using the specification and books
No exam questions are included due to copy right
Including:
Homework booklets
Assessment sheets
Interactive powerpoints (rarely seen in A-level)
RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE
Unit 1 Section 6: 3.1.6 Chemical equilibria, Le Chatelier’s principle and Kc
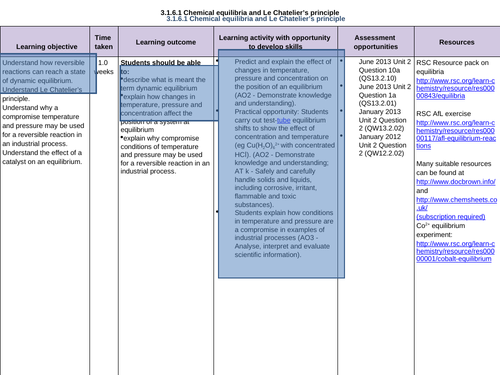
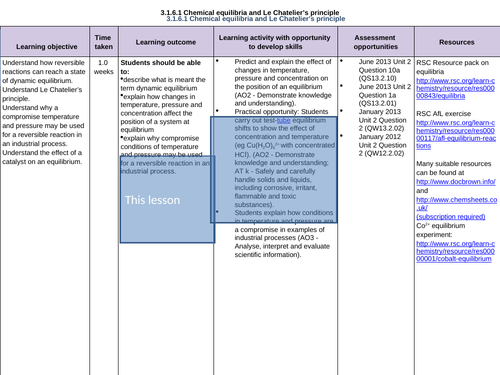
3.1.6.1 Chemical equilibria and Le Chatelier’s principle
LESSON 1:
Objectives:
To understand how reversible reactions can reach a state of dynamic equilibrium
To understand Le Chatelier’s principle
To apply Le Chatelier’s principle to reversible reactions
LESSON 2: Equilibrium and Industry
Objectives:
To explain why compromises are made for the production of ammonia
To explain why compromises are made for the production of Ethanol
To explain why compromises are made for the production of Methanol
Unit 1 Section 6: 3.1.6 Chemical equilibria, Le Chatelier’s principle and Kc 3.1.6.2 Equilibrium constant Kc for homogeneous systems
LESSON 3:
Objectives:
To understand what the Equilibrium Constant, Kc is
To write an expression for Kc including units
To calculate Kc including units
LESSON 4:
Objectives:
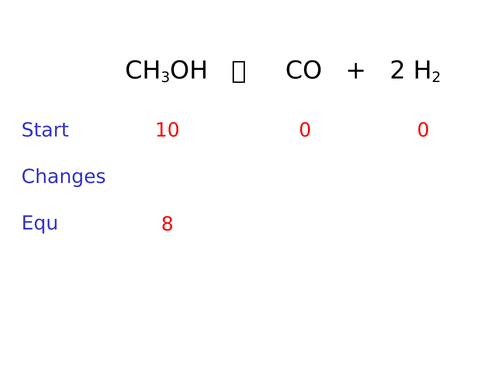
To understand how to work out moles at equilibrium
To calculate Kc after calculating moles
To know how to use Kc to work out the composition of an equilibrium mixture
To know how to use Kc to calculate the amount of reactant needed
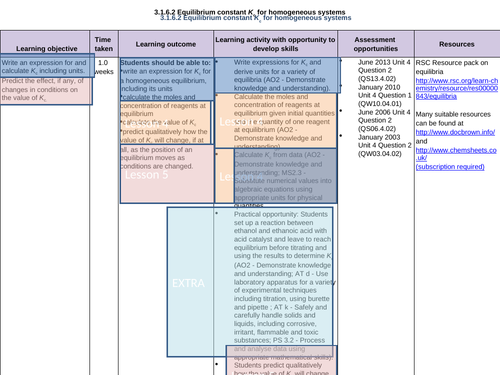
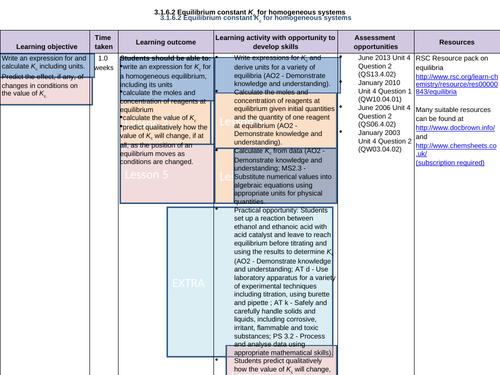
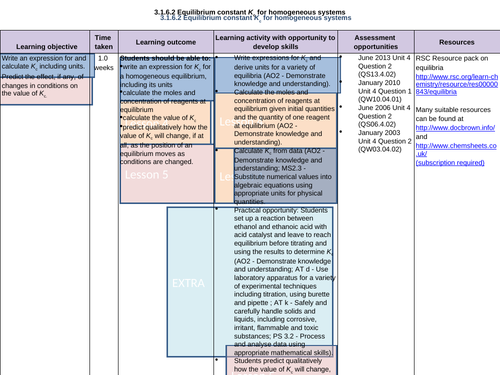
LESSON 5:
Objectives:
To predict the effect, if any, of the changes in conditions on the value of Kc
To predict qualitatively how the value of Kc will change, if at all, as the position of an equilibrium moves as conditions are changed
To consolidate learning
Homework booklet with answers
Leave feedback and enjoy !!
Get this resource as part of a bundle and save up to 41%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
AQA AS and A2 Acid, bases, buffers, Kp, Kc and equilibrium COMPLETE LESSON PACKAGE
AQA AS and A2 Acid, bases, buffers, Kp, Kc and equilibrium COMPLETE LESSON PACKAGE Saving 19%
AQA AS and A2 level Unit 4: COMPLETE LESSONS Equilibria Kc, Kp and Le Chateliers
Bundle for both AS and A2 equilbrium Saving 19%
AQA AS level Unit 1/Section 1: Physical chemistry COMPLETE (lessons and worksheets) - atomic structure, amount of substance, bonding, energetics, kinetics, equilibria, REDOX
AQA AS level Unit 1 Section 1 Atomic structure (atom, electrons, mass spec, ionisation energies) Including: Homework booklets Assessment sheets Interactive powerpoints (rarely seen in A-level) SECTION 1: Atomic structure 1. FUNDAMENTAL PARTICLES - The atom 2. Atomic models (developing ideas from GCSE) 3. Relative mass, relative atomic mass and atomic number 4 Mass spectrometer 5. Mass spectrum analysis - using mass spectra 6. Electron structure - shells and sub-level (s, p, d, f) 7. Ionisation energies - trends and equations SECTION 2: Amount of Substance 14 lessons in total 1.Masses and Mole Part 1 2.Masses and Mole Part 2 3. Moles in solution 4. Ideal Gas equation part 1 5. Ideal Gas equation part 2 - DEMO 6. Calculation of reacting volumes of gas (EXTRA LESSON - removed from spec) 7. Empirical and Molecular formulea 8. Balancing equations and Ionic equations 9. Reacting masses 10. Atom economy and percentage yield 11. EXTRA LESSON - Limiting reagents (student support IF REQUIRED) 12. Standard solutions 13. Titrations 1 14. REQUIRED PRACTICAL 1 Making a standard solution SECTION 3: Bonding 1. Ionic bonding 2. Metallic bonding 3. Covalent bonding 4. Dative covalent (co-ordinate) bonding 5 + 6 Shapes of molecules 7 Electronegativity and bond polarity 8 + 9 Forces acting between molecules (van de Waals, dipole-dipole and Hydrogen bonding) 10 States of matter and a summary of 4 types of crystal structure - molecular, macromolecular, ionic and metallic SECTION 4: Energetics 1. Endothermic/exothermic 2. Measuring q (Measuring Enthalpy Change) 3. PRACTICAL CHOICES 4. Enthalpy of Formation 5. Enthalpy of Combustion 6. Required Practical 7. Bond Enthalpy SECTION 5: KINETICS 1. Collision theory and rates (GCSE RECAP) 2. Maxwell-Boltzmann distribution 1 3. Maxwell-Boltzmann distributions 2 4. REQUIRED PRACTICAL 3 5. Catalysts SECTION 6: Equilibria 1. Dynamic equilibrium + Le Chatelier’s principle 2. Equilibrium and Industry 3. Equilibrium Constant, Kc 4. Kc - calculating moles and composition 5. To predict the effect, if any, of the changes in conditions on the value of Kc SECTION 7: RedOx 1. ‘oxidation’ and ‘reduction’ and oxidation states 2. and 3. 1/2 equations (oxidising agents and reducing agents) 4. Optional practicals FOR MORE INFORMATION SEE EACH INDIVIDUAL UPLOAD Save 37% buying in bulk
AQA AS Level Unit 1 Section 6 + 7 Equilibria + REDOX-Chemical equilibria, Le Chatelier’s principle, oxidation states, oxidation, reduction, ionic and Kc
AQA AS Level Unit 1 Section 6 Equilibria-Catalysts, Chemical equilibria, Le Chatelier’s principle and Kc AQA AS Level Unit 1 Section 7 Oxidation, reduction + REDOX equations unit- Ionic, oxidation states Using the specification and books No exam questions are included due to copy right Including: Homework booklets Assessment sheets Interactive powerpoints (rarely seen in A-level) RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE Unit 1 Section 6: 3.1.6 Chemical equilibria, Le Chatelier’s principle and Kc 3.1.6.1 Chemical equilibria and Le Chatelier’s principle LESSON 1: Objectives: To understand how reversible reactions can reach a state of dynamic equilibrium To understand Le Chatelier’s principle To apply Le Chatelier’s principle to reversible reactions LESSON 2: Equilibrium and Industry Objectives: To explain why compromises are made for the production of ammonia To explain why compromises are made for the production of Ethanol To explain why compromises are made for the production of Methanol Unit 1 Section 6: 3.1.6 Chemical equilibria, Le Chatelier’s principle and Kc 3.1.6.2 Equilibrium constant Kc for homogeneous systems LESSON 3: Objectives: To understand what the Equilibrium Constant, Kc is To write an expression for Kc including units To calculate Kc including units LESSON 4: Objectives: To understand how to work out moles at equilibrium To calculate Kc after calculating moles To know how to use Kc to work out the composition of an equilibrium mixture To know how to use Kc to calculate the amount of reactant needed LESSON 5: Objectives: To predict the effect, if any, of the changes in conditions on the value of Kc To predict qualitatively how the value of Kc will change, if at all, as the position of an equilibrium moves as conditions are changed To consolidate learning AQA AS Level Unit 1 Section 7 Oxidation, reduction + REDOX equations unit- Ionic, oxidation states Using the specification and books No exam questions are included due to copy right Including: Homework booklets Assessment sheets Interactive powerpoints (rarely seen in A-level) RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE Fully explained methods for ionic equations and all answers explained in great detail - all about oxidation states and ionic reactions a large amount of work has been put in to ensure everything is explained to the highest standards. Oxidation, reduction + REDOX equations unit- Ionic, oxidation states: LESSON 1: Objectives: To recap what is meant by ‘oxidation’ and ‘reduction’ To know what an oxidation state is To be able to calculate an oxidation state of an element in a compound LESSON 2 + 3 Objectives: To be able to write half equations from balanced equations To be able to combine half equations to make the overall balanced redox equation To understand the terms oxidising agent and reducing agent LESSON 4: Optional practicals (2p) Homework booklet with answers Leave feedback and enjoy !!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
