


Structure 2 / IB Chemistry / Structure 2.1
+worksheets
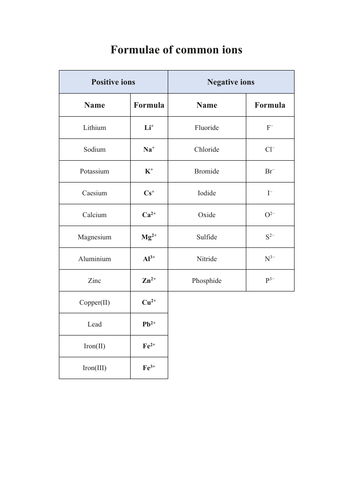
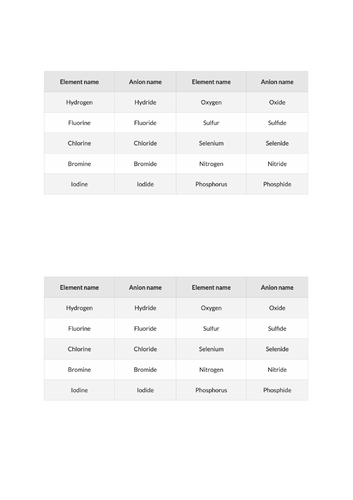
+Formulae of common ions / ionic compounds
Structure 2. Models of bonding and structure
Structure 2.1.1 — When metal atoms lose electrons, they form positive ions called cations. When non-metal atoms gain electrons, they form negative ions called anions.
Structure 2.1.2 — The ionic bond is formed by electrostatic attractions between oppositely charged ions.
Structure 2.1.3—Ionic compounds exist as three-dimensional lattice structures, represented by empirical formulas.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.