
A structured KS5 lesson including starter activity and AfL work tasks and main work tasks on Transition Metals & Redox Reactions. All tasks have worked out answers, which will allow students to self assess their work during the lesson
By the end of this lesson KS5 students should be able to:
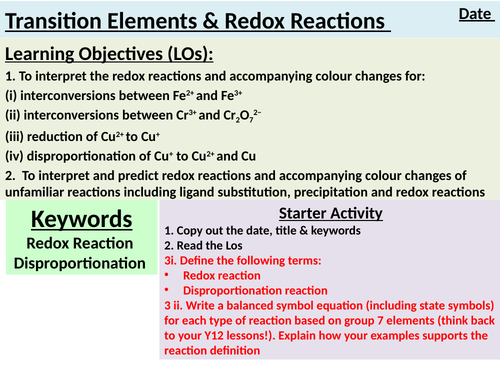
LO1. To interpret the redox reactions and accompanying colour changes for:
(i) interconversions between Fe2+ and Fe3+
(ii) interconversions between Cr3+ and Cr2O72−
(iii) reduction of Cu2+ to Cu+
(iv) disproportionation of Cu+ to Cu2+ and Cu
LO2. To interpret and predict redox reactions and accompanying colour changes of unfamiliar reactions including ligand substitution, precipitation and redox reactions
NOTE: 23 printable flashcards of all the transition element reactions: precipitation, ligand substitution and redox reactions is available here
https://www.tes.com/teaching-resource/resource-12637622
Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons including using your own lesson PowerPoints is a fundamental skill of a qualified/unqualified teacher that will be reviewed during these scenarios outlined above
Something went wrong, please try again later.
Very good. I love it. It is clear and filled with questions. Thank you for sharing
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.