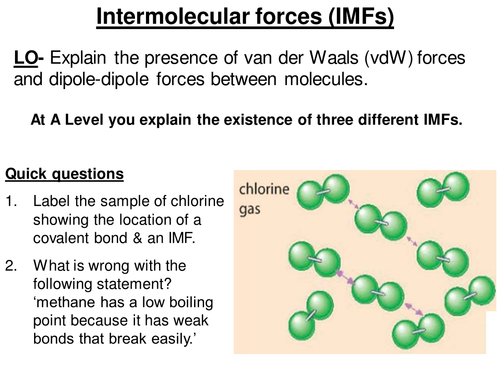
There is first a basic review of intermolecular forces from GCSE to ensure students have understood this often misconceived concept. The lesson then focuses on van der Waals forces by looking at helium as an example to illustrate the idea.
Students then complete application questions referring to the trend in the boiling points of the remaining noble gases as well as the halogens.
The lesson then moves on to dipole-dipole forces by using hydrogen chloride as an example.
Students then complete some summary application questions to review their learning from the lesson.
Students then complete application questions referring to the trend in the boiling points of the remaining noble gases as well as the halogens.
The lesson then moves on to dipole-dipole forces by using hydrogen chloride as an example.
Students then complete some summary application questions to review their learning from the lesson.
Get this resource as part of a bundle and save up to 33%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£2.00