







AQA A2 Level 3.2.5 Transition metals and 3.2.6 Reactions of ions in aqueous solution COMPLETE LESSON PACKAGE plus practicals and required practicals
Using the specification and books
No exam questions are included due to copy right
Including:
Homework booklets
Assessment sheets
Interactive powerpoints (rarely seen in A-level)
You will need a membership to Chemsheets - doesn’t have to be used with chemsheets
RSC STARTER FOR 10 CAN BE FOUND ON RSC WEBSITE
Very detailed- will not suit everyone (hidden slides are extras depending on ability of class)
3.2.5 Transition metals
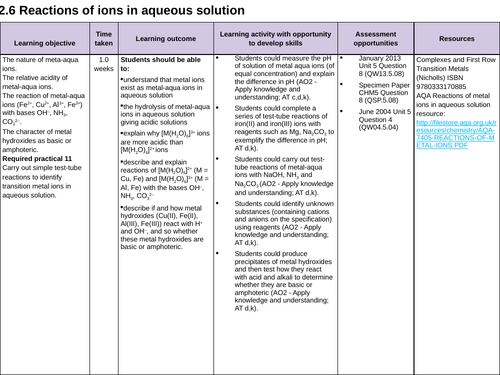
3.2.6 Reactions of ions in aqueous solution
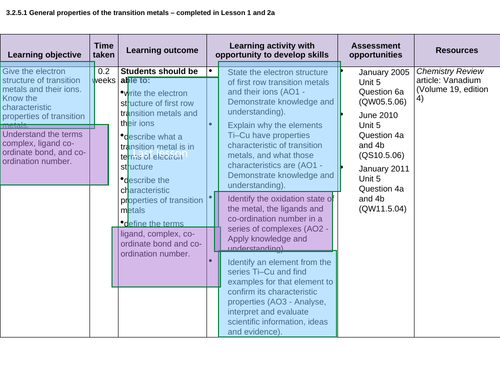
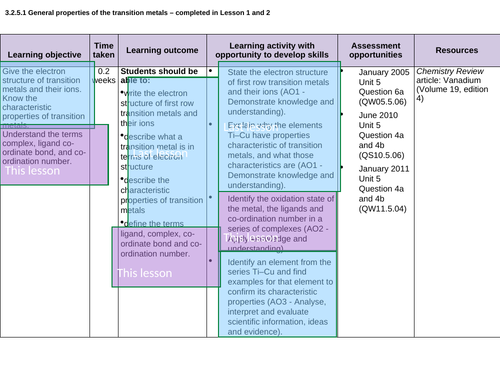
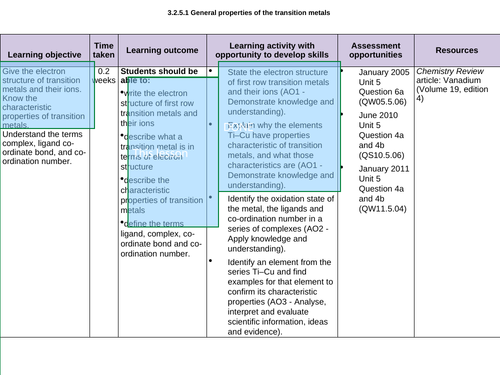
3.2.5.1 General properties of the transition metals – LESSON 1 and LESSON 2 crossover (see each ppt for allocation)
3.2.5.3 Shapes of complex ions (done before subsitution reactions – starts in lesson 2)
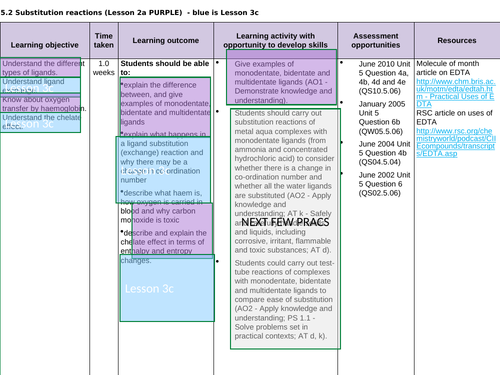
3.2.5.2 Substitution reactions (covered all in lesson 3a)
3.2.5.4 Formation of coloured ions
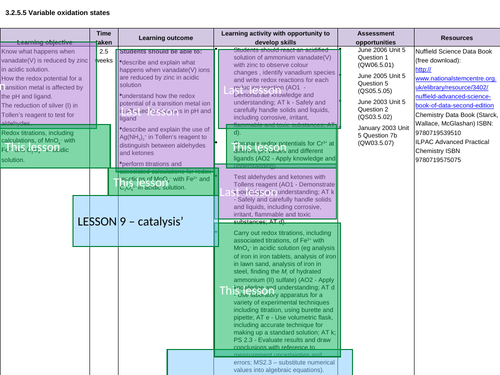
3.2.5.5 Variable oxidation states
3.2.5.6 Catalysts
3.2.6 Reactions of ions in aqueous solution
Lesson 1: General properties of TM
To write the electron structure of first row transition metals and their ions and describe what a transition metal is in terms of electron structure
That these characteristics include complex formation of coloured ions, variable oxidation state and catalytic activity
Lesson 2: TM complexes and ligands
To define the terms ligand, complex, co-ordinate bond and co-ordination number
Explain the difference between and give examples of monodentatate, bidentate and multidentate ligands.
To identify the oxidation state of the metal and understand the term co-ordination number
To know about oxygen transfer by haemoglobin
Lesson 3: Shapes of TM complexes
To give examples of and sketch the shapes of octahedral, tetrahedral, square planar and linear complexes
To understand how to name complexes
To know how some complexes can show cis-trans (E–Z) or optical isomerism
To know the complexes in cisplatin and Tollen’s reagent.
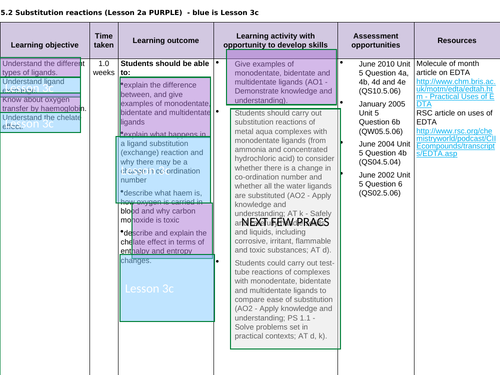
Lesson 3c: Ligands subsitution
To understand ligand exchange and explain why there may be a change in co-ordination number
To describe and explain the chelate effect in terms of entropy and enthalpy
To complete a series of exam questions to consolidate
To complete a series of practical sessions to consolidate (see prac. requirements ->)
Lesson 3d Practical - Ligand displacement series
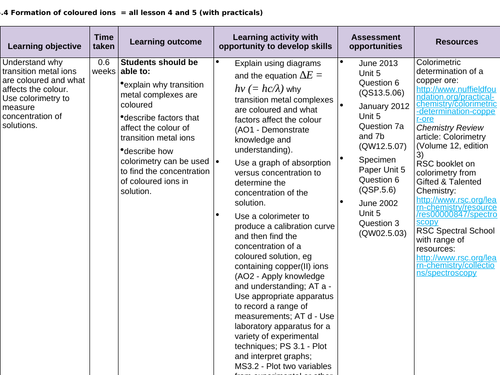
Lesson 4 + 5: Coloured ions
To describe factors that affect the colour of TM ions
To explain why TM complexes are coloured
To describe how colorimetry can be used to find the concentration of coloured ions in solution
Lessons 5b: Practical - Determining the formula of a complex ion
Lesson 6: Variable os
Lesson 6b: Practicals
Lesson 7: Redox titrations
Lesson 7b Practical
Lesson 8: Practical
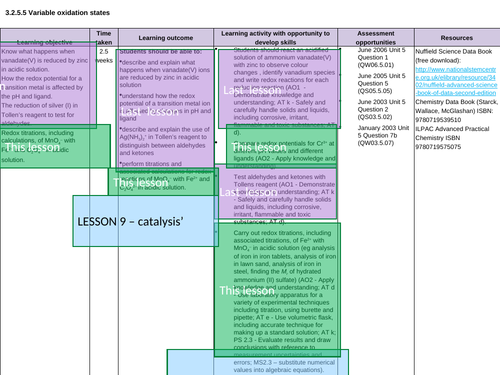
Lesson 9: Catalysts
Lesson 9b: Practical
Lesson 9bi: Practical
Lesson 10, 11, 12: Reactions of ions in aqueous solution
Lesson 13: REQUIRED PRACTICAL 11
Homework booklets
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.