

This lesson continues with bonding- focusing on Covalent Bonding.
This lesson covers the spec:
Definition of Covalent Bonding. State that non-metallic elements form non-ionic compounds using a different type of bonding called covalent bonding involving shared pairs of electrons.
Draw dot-and-cross diagrams to represent the sharing of electron pairs to form single covalent bonds in simple molecules, exemplified by H2, Cl2, H2O, CH4 and HCl.
Draw dot-and-cross diagrams to represent the multiple bonding in N2, C2H4 and CO2 *
Again as with the previous lesson I find this a curious place in the course to deal with bonding - before having gone through the periodic table of elements. The previous lessons does deal with orbitals and electron configuration , but logically I would prefer to move onto the Periodic Table after this. Anyway I have tried to also explain a little about classifying materials and the periodic table to make it relevant.
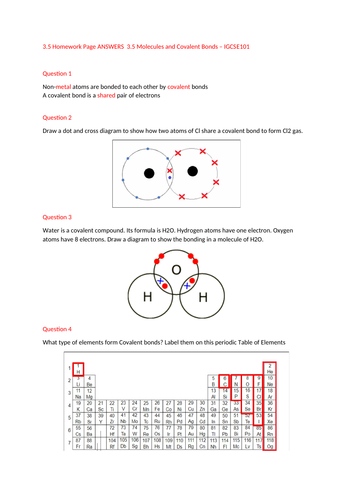
The PowerPoint goes over the basics as per the Spec but also included is a work sheet/homework page with solutions for the students to work on.
My advice is to practice these dot and cross diagrams as much as possible and at the end of this lesson students should be able to draw the bonds between H2, Cl2, H2O, CH4 and HCl.
Then when it comes to learning about the Periodic Table it will fall into place!
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.