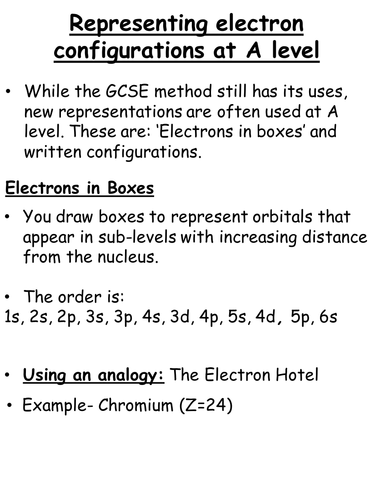
This activity introduces electron configurations at A Level by using the 'electron hotel' as an analogy.
Students follow a given set of rules to assign electrons (the people) to their orbitals (rooms) with increasing distance from the nucleus (ground floor).
Once students can competently use the electrons in boxes method, they then move on to writing electron configurations.
Answers for all examples and questions are included also.
Students follow a given set of rules to assign electrons (the people) to their orbitals (rooms) with increasing distance from the nucleus (ground floor).
Once students can competently use the electrons in boxes method, they then move on to writing electron configurations.
Answers for all examples and questions are included also.
Something went wrong, please try again later.
The chromium example is wrong, the 3d orbital is filled to 3d5 and 4s1, this is only for chromium and copper (3d10 and 4s1)
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£1.00