

LESSON OBJECTIVE: Understand oxidation and addition polymerisation reactions with alkenes and describe the use and pollution of plastics.
In this lesson we continue our investigation of alkenes by discussing the different products that can be formed in the oxidation of an alkene, introduce the concept of addition polymerisation to make polymers and how these polymer plastics can be used and the pollution associated with them. This is lesson 7 in our organic chemistry series of Unit 15: Hydrocarbons (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Learning Outcomes:
(taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum)
15.2 Alkenes
a) describe the chemistry of alkanes as exemplified, where relevant, by the following reactions of ethene and propene (including the Markovnikov addition of asymmetric electrophiles using propene as an example):
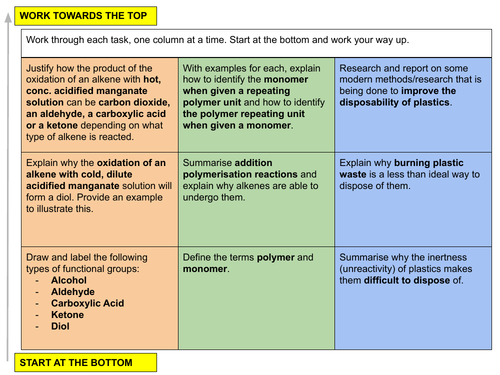
(ii) oxidation by cold, dilute, acidified manganate(VII) ions to form the diol
(iii) oxidation by hot, concentrated, acidified manganate(VII) ions leading to the rupture of the carbon-carbon double bond in order to determine the position of alkene linkages in larger molecules
(iv) polymerisation
d) describe the characteristics of addition polymerisation as exemplified by poly(ethene) and PVC
e) deduce the repeat unit of an addition polymer obtained given a monomer
f) identify the monomer(s) present in a given section of an addition polymer molecule
g) recognise the difficulty of the disposal of poly(alkane)s, i.e. non-biodegradability and harmful combustion products
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.