


These PowerPoints were planned as part of the IB scheme of work on Structure and Bonding and cover the necessary content for both the Standard and Higher Level topics. They would also be suitable for other post-16 courses.
Included are fully completed PowerPoints, student versions of the PowerPoints with sections to complete independently and some exam style questions.
Topics included are:
Ionic Bonding
What is ionic bonding?
Common positive and negative ions
Working out the formula of ionic compounds
Giant ionic lattices
Properties of ionic substances
Covalent Bonding
What is covalent bonding?
How to draw Lewis structures
How to tell if a substance will be ionic or covalent
The Octet rule and how it can be broken
Coordinate bonds and compounds which contain them
Resonance structures
VSEPR theory
Shapes of molecules with up to 6 bonding pairs
Shapes of molecules with up to 6 bonding and lone pairs
Giant covalent bonding - diamond, graphite and silica
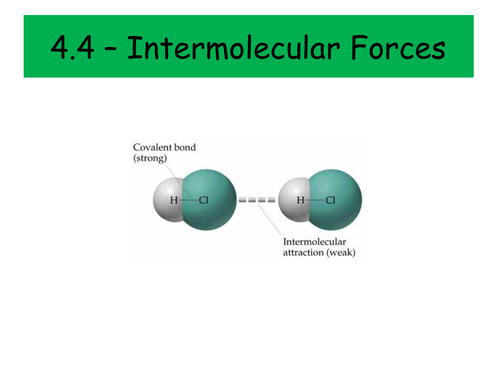
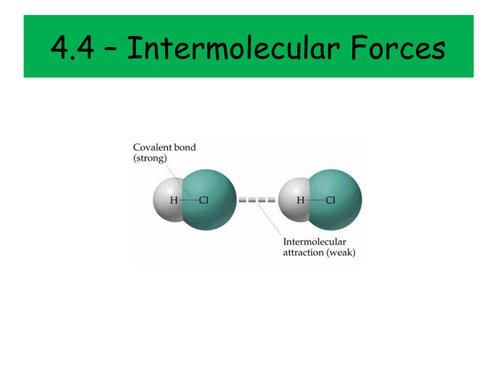
Intermolecular Bonding
- London forces
- Permanent dipole-permanent dipole forces
- Permanent dipole-induced dipole forces
- Hydrogen bonding
- Solubility and intermolecular forces
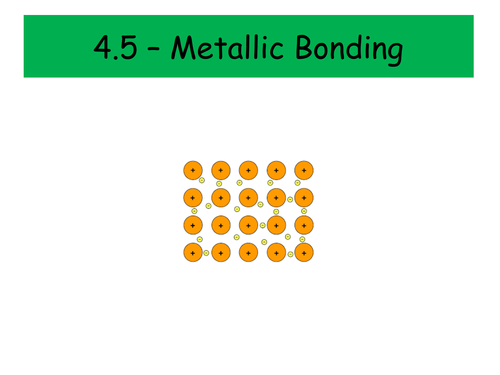
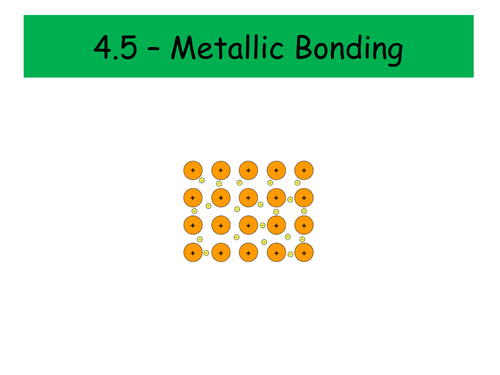
Metallic Bonding
How do we describe a metallic structure?
How to predict which metal will have the high melting point
Properties of metals
Properties of alloys
Advanced covalent bonding, electron domains and molecular geometries
Assigning formal charge
Exceptions to the octet rule
Formation of sigma and pi bonds
The composition of single, double and triple bonds
Resonance hybrids and delocalisation
The structure of benzene - Kekule and delocalised
Absorption of UV light in the atmosphere
Catalysis of ozone depletion by CFCs and NOx gases
Hybridisation
sp3, sp2, sp hybridisation: how it happens, resulting shapes and how to identify molecules with each type of hybridisation.
Get this resource as part of a bundle and save up to 25%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
A great resource. I tweak a few parts (hybridisation- trigonal pyramidal is not the same as trigonal planar) but generally excellent. Thank you!
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.