Mass Spectrometry A level Chem lesson with questions and answers
<p>Mass spectrometry principle A level Chemistry, including mass spectrometry problems and solutions, and mass spectrometry Save My Exams examples and diatomic molecules.</p>
<p><strong>All the slides in this lesson are fully animated and include answers to every mini plenary question and exam question.</strong></p>
<p>The breakdown of the slides is as follows:</p>
<p>Slide 1 - Title and 5-minute starter. The starter is a grid of four questions entitled ‘last week, last lesson, today’s learning and future learning’. Use this generic slide for all of your lessons by simply changing the questions and the answers each time.<br />
Slide 2 - Lesson objectives (see above)<br />
Slide 3 explanation of the term ‘relative atomic mass’ by means of a picture (dual coding)<br />
Slides 4 – written definitions of both relative atomic mass and relative isotopic mass<br />
Slide 5 – introduction to the mass spectrometer<br />
Slide 6 – photo of an actual mass spectrometer<br />
Slide 7 – short, embedded video, explaining the principle of the mass spectrometer<br />
Slide 8 – labelled diagram of a mass spectrometer which closely resembles what a student might see in an exam<br />
Slides 9 – 12 – outline of how a mass spectrometer works, from ionisation, acceleration, deflection and detection<br />
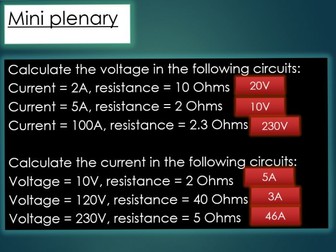
Slides 13 - 15 – mass spectrometry of an element, with worked examples and <strong>mini plenary questions on calculating relative atomic mass from a mass spectrum with answers</strong><br />
Slide 16 – 19 – this section deals with mass spectrometry of diatomic molecules, namely chlorine. Students are introduced to the concept of the unfragmented molecular ion and explanation of how this produces the peaks at 70, 72 and 74 are offered. The math behind the ratios (9:6:1) is also explained<br />
Slides 20 – 22 – this last section of teaching covers mass spectrometry of polyatomic molecules. For the Edexcel specification, students only need to be aware of the significance of the M as the relative molecular mass and the M+1 peak being caused by C-13. Slide 22 has an image of the mass spectrum of pentane, showing clearly the M peak.<br />
Slide 23 – Exam questions slide<br />
Slides 24 – 28 – <strong>mark scheme answer for each question as you click!</strong></p>
<p>If you have a positive experience with the resource please leave a positive review!</p>