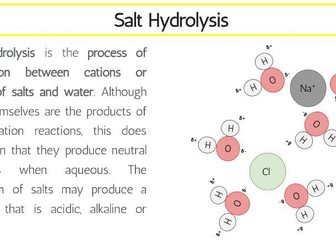
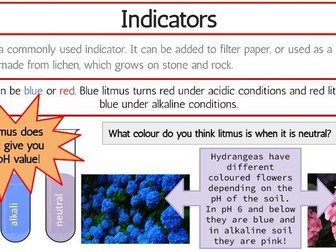
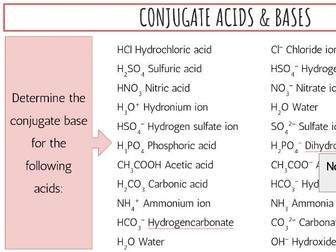
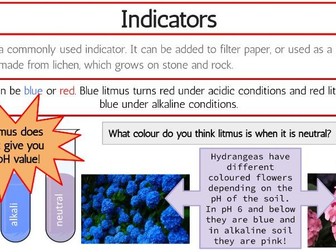
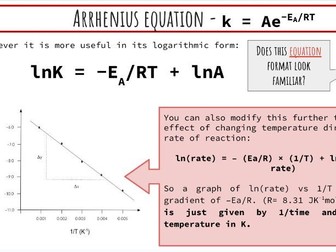
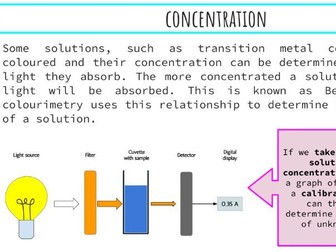
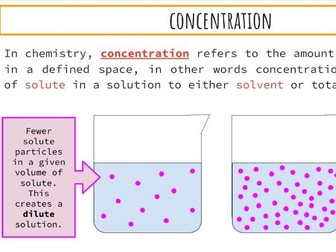
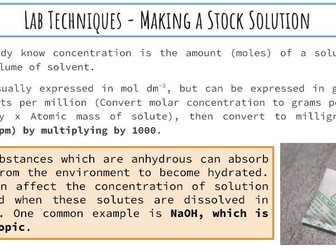
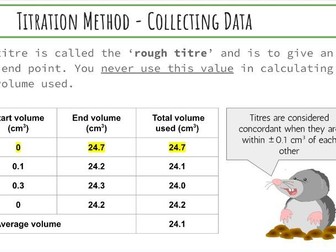
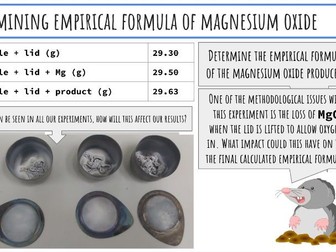
IB Chemistry Structure 1 Workbook
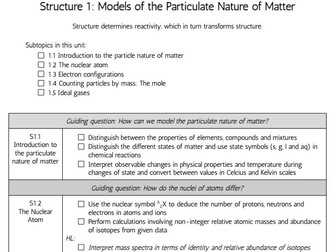
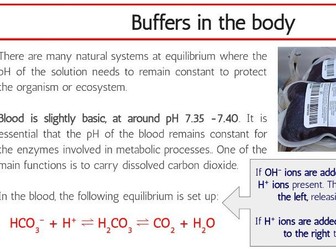
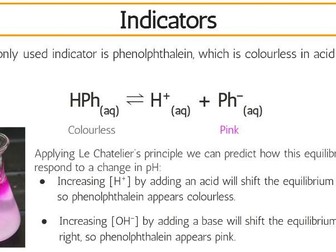
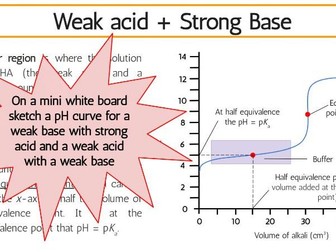
This complete workbook for structure 1 provides a comprehensive blend of class tasks, practical experiments, and exam-style questions to reinforce learning and ensure thorough preparation for assessments. The workbook includes key content aligned with the IB Chemistry syllabus, ensuring that students can build a strong foundation in this unit.