

















AQA GCSE 9-1 CHEMISTRY UNIT 3.3 Yield + atom economy + percentage yield, molar gas, conc TRIPLE
4.3.3 Yield and atom economy of chemical reactions (chemistry only)
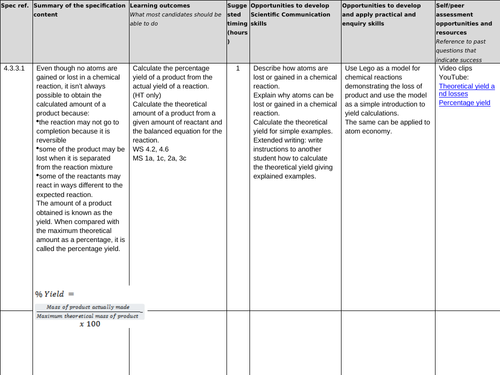
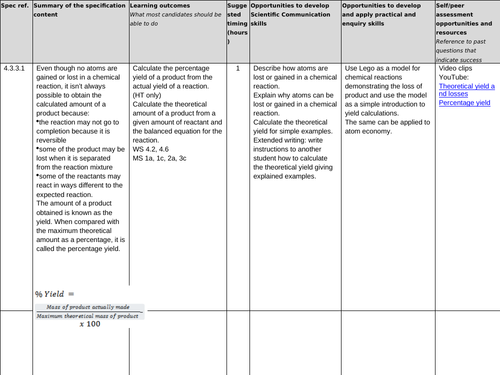
4.3.3.1 Percentage yield
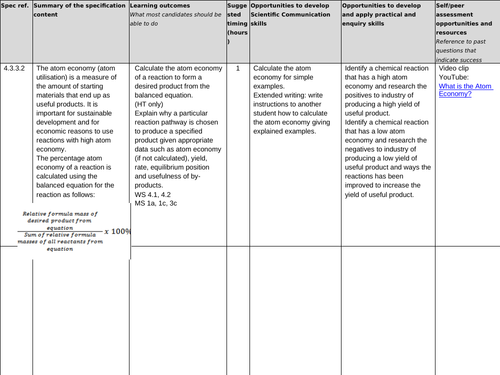
4.3.3.2 Atom economy
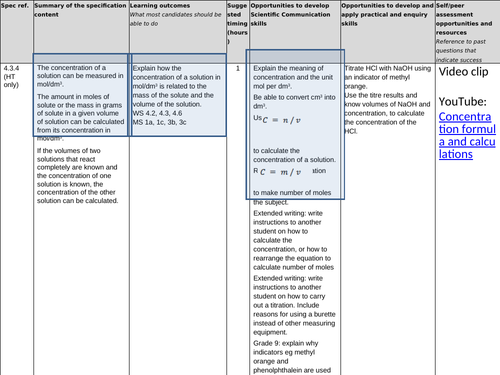
4.3.4 Using concentrations of solutions in mol/dm3 (chemistry only)
(HT only)
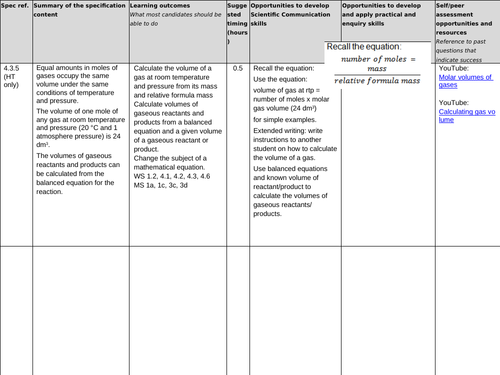
4.3.5 Use of amount of substance in relation to volumes of gases
(chemistry only) (HT only)
For combined HIGHER lessons - https://www.tes.com/teaching-resource/aqa-gcse-9-1-chemistry-unit-3-2-amount-of-substance-higher-moles-limiting-reagent-12167323
For combined lessons - https://www.tes.com/teaching-resource/aqa-gcse-9-1-chemistry-unit-3-1-chemical-measurements-conservation-of-mass-equations-no-moles-12167318
Content split over 6 lessons (lessons in our school are 40 minutes so can condense material for longer lessons if required)
All exam questions have been removed for copyright purposes
All extension questions available on each slide
Answers all underneath each slide
Support also available where necessary
AfL sections and mini quizzes
Reducing the need for photocopying
Homework
Homework can also be used as extension sheets in lessons - or for higher ability students
Lesson 1: Percentage yield
To understand the difference between the actual yield and the theoretical yield
To be able to calculate the percentage yield of a reaction from the actual yield and the theoretical yield
To consolidate learning with questions
Lesson 2: Atom economy
To recall the atom economy of a reaction
To make Magnesium sulphate in 3 different ways then work out which is the best (most economical!)
To explain why a particular reaction pathway is chosen to produce a particular product, given data (H)
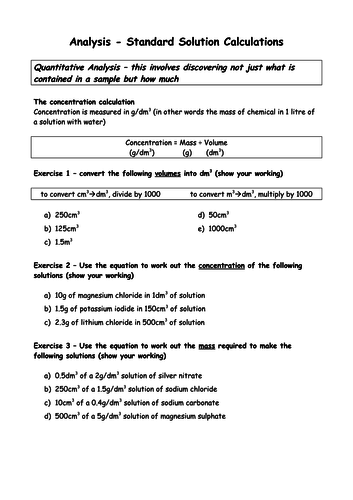
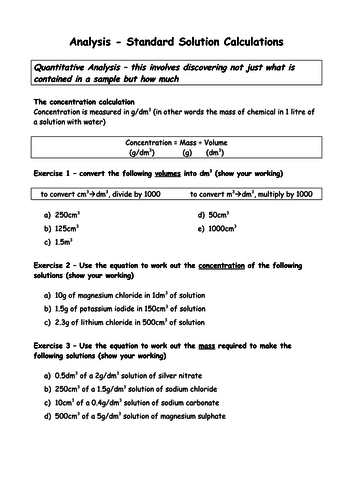
Lesson 3 & 4: Concentration
STARTER: To recap how to interconvert between cm3 and dm3 and to work out the concentration equation
To recap how to calculate concentrations in g dm-3 (H)
To be able to calculate concentrations in mol dm-3 (H)
To understand how to interconvert between mol dm-3 and g dm-3 (H)
Lesson5 & 6: Molar volume of gas
To know how to define molar volume of gases at room temperature and pressure
To be able to use the molar volume in calculations involving the masses of solids and volumes of gases
To understand how to use Avogadro’s law to calculate volumes of gases involved in gaseous reactions.
Get this resource as part of a bundle and save up to 26%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.