This lessons is designed to run through extension additional content quickly to a high achieving group. Students are re-introduced to electrolysis. Students fill in the A3 sheet throughout the class discussion.
Lesson 1
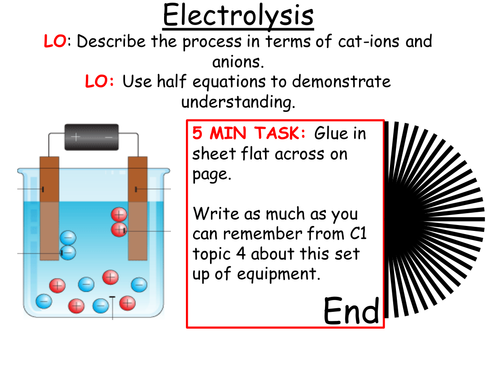
-electrolysis as decomposition.
-Cathode attracts positive cations because they are negatively charged.
-anode attracts negative anions because they are positively charged.
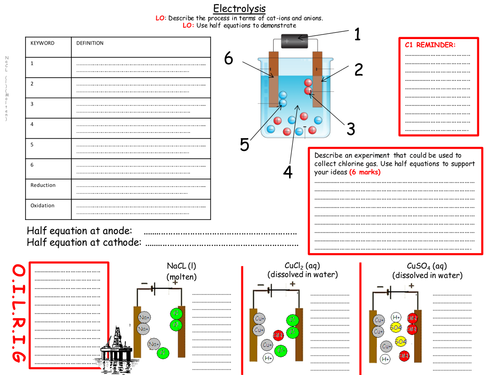
-OILRIG -oxidation is loss (and) reduction is gain (of electrons)
Lesson 2
-Students are introduced to the differences of electrolysing molten materials and solutions containing different solutes.
-Students complete a practical experiment: Electroplating a iron nail with copper. -Students complete exam questions.
Lesson 1
-electrolysis as decomposition.
-Cathode attracts positive cations because they are negatively charged.
-anode attracts negative anions because they are positively charged.
-OILRIG -oxidation is loss (and) reduction is gain (of electrons)
Lesson 2
-Students are introduced to the differences of electrolysing molten materials and solutions containing different solutes.
-Students complete a practical experiment: Electroplating a iron nail with copper. -Students complete exam questions.
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have purchased this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£4.00