Energy (OCR A Level Chemistry)
6 Full Lesson Bundle covering the first 6 chapters in the OCR A Level Chemistry Chapter on Energy
Lesson 1: Lattice Enthalpy
**By the end of the lesson students will:
1. Explain the term lattice enthalpy
2. Understand the factors that determine the size of lattice enthalpy
3. Explain the terms standard enthalpy change of formation and first ionisation energy**
Lesson 2: Born-Haber Cycles
**By the end of the lesson students will:
**1. Construct Born Haber Cycle diagrams for ionic compounds from enthalpy change values
**2. Calculate the value for lattice enthalpy from Born Haber Cycle diagrams
**3. Calculate other enthalpy change values from Born Haber Cycle diagrams
Lesson 3: Enthalpy Changes of Solution & Hydration
**By the end of the lesson students will:
**1. Define the terms enthalpy change of solution and hydration
**2. Construct enthalpy cycles using the enthalpy change of solution of a simple ionic solid
**3. Qualitatively explain the effect of ionic charge and ionic radius on the exothermic value of lattice enthalpy and enthalpy change of hydration**
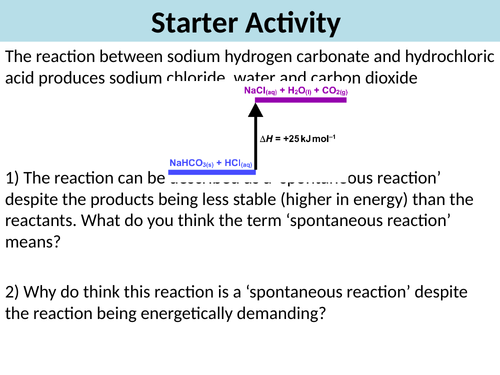
Lesson 4: Entropy
**By the end of lesson students will:
**1. Know that entropy is a measure of the dispersal of energy in a system, which is greater the more disordered a system
**2. Explain the difference in entropy of solids, liquids and gases
**3. Calculate the entropy change of a reactant based on the entropies provided for the reactants and products
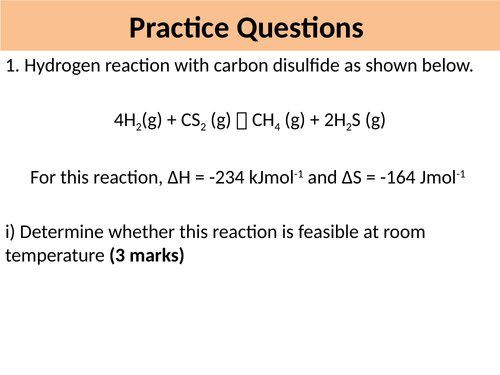
Lesson 5: Gibbs Free Energy (Part 1)
**By the end of the lesson students will:
**1. Explain that the feasibility of a process depends upon ΔG being negative which in turn depends upon ΔS, ΔH and the T of the system
**2. Recall the Gibbs’ Equation and calculate ΔG, ΔH, ΔS or T
**3.Calculate ΔG, ΔH, ΔS or T using the Gibbs’ Equation
Lesson 6: Gibbs Free Energy (Part 2)
By the end of the lessons students will:
**1. Explain that the feasibility of a process depends upon ΔG being negative which in turn depends upon ΔS, ΔH and the T of the system
2. Recall the Gibbs’ Equation and calculate ΔG, ΔH, ΔS or 3. Calculate ΔG, ΔH, ΔS or T using the Gibbs’ Equation**
The teacher will be able to check students have met these learning objectives through starter activities, discussion questions, mini AfL tasks and practice questions for students to complete
***Declaimer: Please refrain from purchasing this popular resource for an interview lesson or a formal observation. This is because planning your own lessons, including using your own lesson PowerPoints, is a fundamental skill of a qualified/unqualified teacher that will be assessed during the scenarios outlined above***