This lesson is designed for the NEW AQA Chemistry GCSE, particularly the 'Atomic Structure & Periodic Table' SoW.
For more lessons designed to meet specification points for the NEW AQA Trilogy specifications for Biology, Chemistry and Physics please see my shop: https://www.tes.com/teaching-resources/shop/SWiftScience
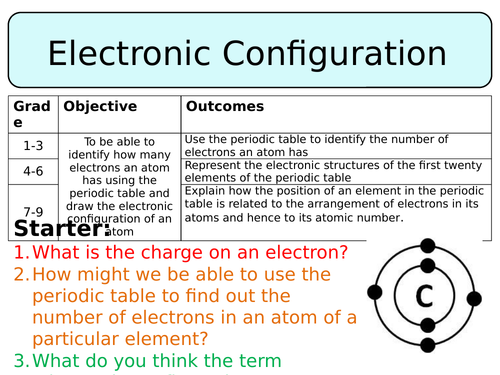
First task is a true or false task about the structure of an atom just to remind pupils about what they already know about these sub-atomic particles.
Then pupils are played a video about the rules regarding electronic configuration, with which they should fill in a worksheet, either completing sentences or answering questions. Once this is finished pupils will self-assess their work. Next, the rules of how many electrons each shell can hold is demonstrate using diagrams, pupils are given examples to further consolidate this information.
Next, pupils are given a table with different elements listed, they will need to identify the mass number, atomic number, draw and write out the electronic configuration for each element. This can be assessed once it has been completed. The next task is an extension of what has just been completed, pupils are given a worksheet where they need to fill in the electron shells for the first 20 elements as well as write out the electronic configuration. Again, pupils will be provided with the answers to mark this work.
The final two activities focuses on the importance of how many electrons are in the outer shell of an atom of an element and what this means for the reactivity of this element. Pupils will watch a further video and also complete fill-in-the-blank sentences to summarise what they have learnt.
The plenary task is a set of graded questions about atomic structure.
All resources are included at the end of the presentation. Thanks for looking, if you have any questions please let me know in the comments section and any feedback would be appreciated :)
For more lessons designed to meet specification points for the NEW AQA Trilogy specifications for Biology, Chemistry and Physics please see my shop: https://www.tes.com/teaching-resources/shop/SWiftScience
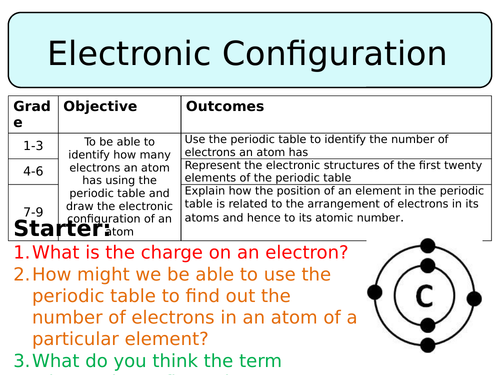
First task is a true or false task about the structure of an atom just to remind pupils about what they already know about these sub-atomic particles.
Then pupils are played a video about the rules regarding electronic configuration, with which they should fill in a worksheet, either completing sentences or answering questions. Once this is finished pupils will self-assess their work. Next, the rules of how many electrons each shell can hold is demonstrate using diagrams, pupils are given examples to further consolidate this information.
Next, pupils are given a table with different elements listed, they will need to identify the mass number, atomic number, draw and write out the electronic configuration for each element. This can be assessed once it has been completed. The next task is an extension of what has just been completed, pupils are given a worksheet where they need to fill in the electron shells for the first 20 elements as well as write out the electronic configuration. Again, pupils will be provided with the answers to mark this work.
The final two activities focuses on the importance of how many electrons are in the outer shell of an atom of an element and what this means for the reactivity of this element. Pupils will watch a further video and also complete fill-in-the-blank sentences to summarise what they have learnt.
The plenary task is a set of graded questions about atomic structure.
All resources are included at the end of the presentation. Thanks for looking, if you have any questions please let me know in the comments section and any feedback would be appreciated :)
Get this resource as part of a bundle and save up to 50%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
Thanks
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.
£3.00