
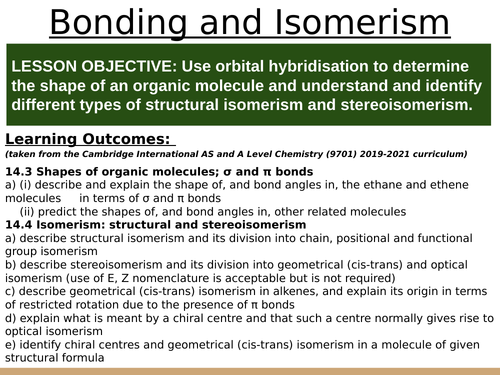
LESSON OBJECTIVE: Use orbital hybridisation to determine the shape of an organic molecule and understand and identify different types of structural isomerism and stereoisomerism.
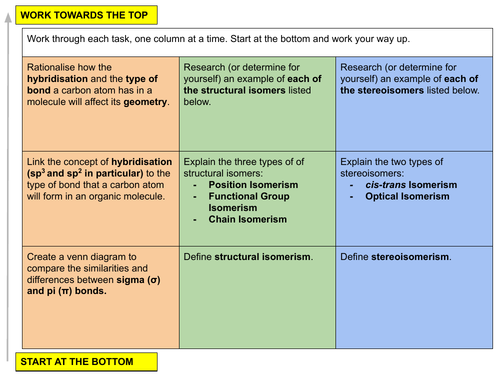
In this lesson we discuss how hybridisation, molecular geometries and sigma and pi bonds are linked to organic structure and formulas. We then introduce isomerism by looking at the different types of structural isomerism (position, functional group and chain) and stereoisomerism (cis-trans and optical). This is lesson two in our organic chemistry unit for Unit 14: An introduction to organic chemistry (from the Cambridge International AS Chemistry Curriculum (9701) 2019-2021 curriculum).
Learning Outcomes:
(taken from the Cambridge International AS and A Level Chemistry (9701) 2019-2021 curriculum)
14.3 Shapes of organic molecules; σ and π bonds
a) (i) describe and explain the shape of, and bond angles in, the ethane and ethene molecules a) in terms of σ and π bonds
(ii) predict the shapes of, and bond angles in, other related molecules
14.4 Isomerism: structural and stereoisomerism
a) describe structural isomerism and its division into chain, positional and functional group isomerism
b) describe stereoisomerism and its division into geometrical (cis-trans) and optical isomerism (use of E, Z nomenclature is acceptable but is not required)
c) describe geometrical (cis-trans) isomerism in alkenes, and explain its origin in terms of restricted rotation due to the presence of π bonds
d) explain what is meant by a chiral centre and that such a centre normally gives rise to optical isomerism
e) identify chiral centres and geometrical (cis-trans) isomerism in a molecule of given structural formula
Something went wrong, please try again later.
This resource hasn't been reviewed yet
To ensure quality for our reviews, only customers who have downloaded this resource can review it
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.