
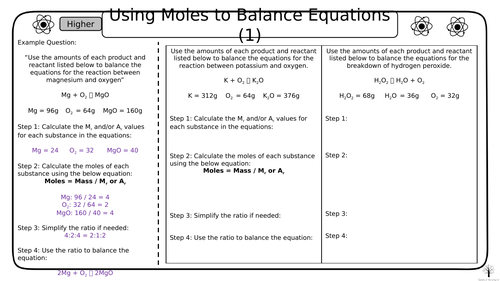
This resource contains 4 worksheets for balancing equations using moles that can be used in class or as homework to enable your students to practice what they have learnt in the classroom.
Students will be:
Using the mole equation
Balancing equations using moles
Calculating the relative molecular and atomic mass of compounds
Using and simplifying ratios
We have worksheets for the following topics in Chemistry Paper 1:
Atomic Structure and The Periodic Table
Atoms, Elements and Compounds
Mixtures and Separation Techniques
Relative Atomic Mass and Electronic Structure
The Periodic Table and Metals
Groups 0 & 1
Group 7
Bonding, Structure, and The Properties of Matter
Ionic Bonding
Covalent and Metallic Bonding
Structure and Bonding of Carbon
Quantitative Chemistry
Balancing Equations
% Mass
Relative Atomic and Formula Mass
Amount of Substances in Equations
The Mole
Using Moles to Balance Equations
Limiting Reactants
Concentration of Solutions
Chemical Changes
Metal Oxides and Reactivity Series
Displacement Reactions
Extraction of Metals and Reduction
Ionic Equations
Reactions of Metals with Acids
Neutralisation of Acids
pH Scale, Neuralisation, and Strong & Weak Acids
Electrolysis
Energy Changes
Energy Transfers and Reaction Profiles
Energy Changes of Reactions
More worksheets will be added in the future.
Please rate and review this resource. Thank you!
Get this resource as part of a bundle and save up to 65%
A bundle is a package of resources grouped together to teach a particular topic, or a series of lessons, in one place.
Something went wrong, please try again later.
Great resource!
I absolutely love this resource, it was great practice for my Y10 set 1s. However, there s a small error on sheet 3 question 1 (balancing should be 1:5:3:4, masses of C3H8 = 44g O2 = 160g CO2 = 132g H2O = 72g Also, on sheet 4, for the final question, the mass of Na2SO4 should be 852g. All spotted by my students. Thanks!
Report this resourceto let us know if it violates our terms and conditions.
Our customer service team will review your report and will be in touch.