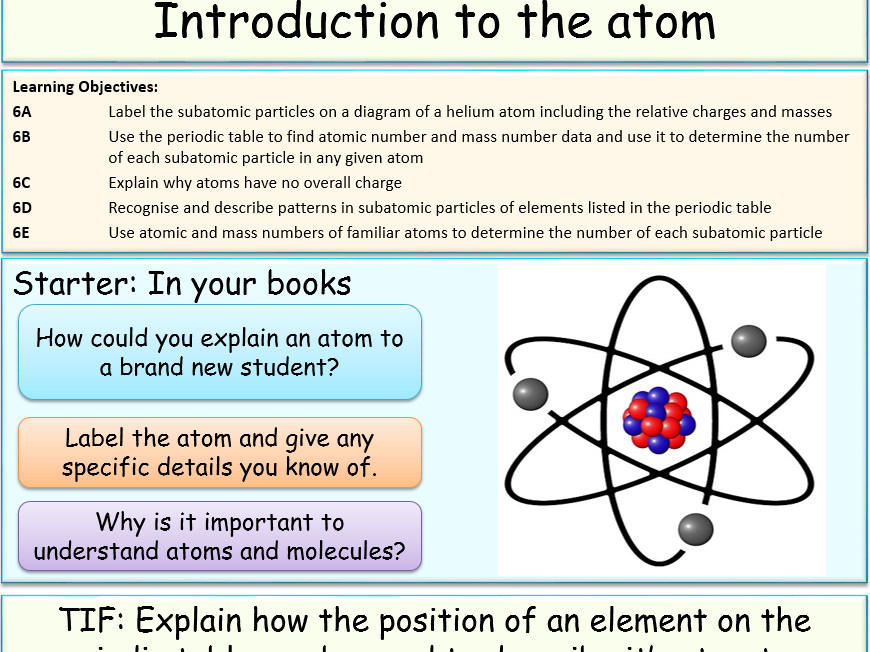
Jubblord's Shop
Supplying secondary and six form science lessons and resources. Covering Biology, Chemistry and Physics. All resources are designed for the latest specifications (AQA and Edexcel). Author holds MChem in Medicinal Chemistry and Pharmacology, qualified and published Analytical chemist and also holds full teaching qualifications.