484Uploads
144k+Views
63k+Downloads
All resources

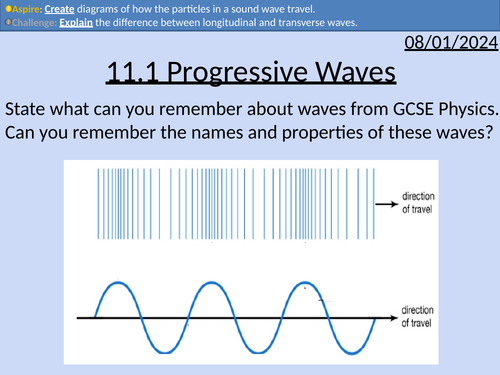
OCR AS level Physics: Progressive Waves
OCR AS level Physics: Progressive Waves is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

OCR AS level Physics: Einstein's Photoelectric Equation
OCR AS level Physics: Einstein’s Photoelectric Effect Equation is a part of the Module 4: Electrons, Waves, and Photons.
Full lesson PowerPoint with worked examples and homework with complete worked answers.
The photoelectric equation
Work function and Kinetic Energy
Determining work function from a graph
Determining threshold frequency from a from graphical analysis.
Determining Plank’s constant from graphical analysis.

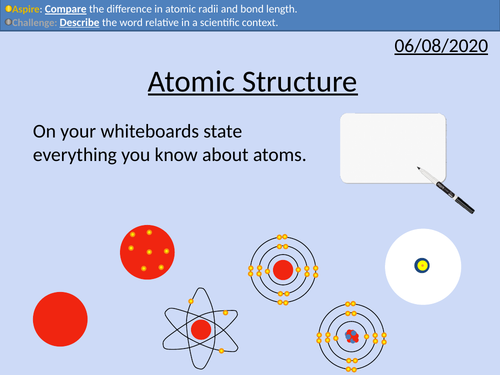
GCSE Chemistry: Atomic Structure
This PowerPoint presentation with worked examples and student questions covers:
• Scientific models as a concept
• Structure of the atom
• Relative mass and charge of subatomic particles
• Bond length of atoms and molecules

OCR Applied Science: 1.3 Ionic and Covalent Bonding
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.3 of Science Fundementals of the OCR Applied Science Spec.
Elements react together to form compounds by i.e.
ionic bonding
covalent bonding

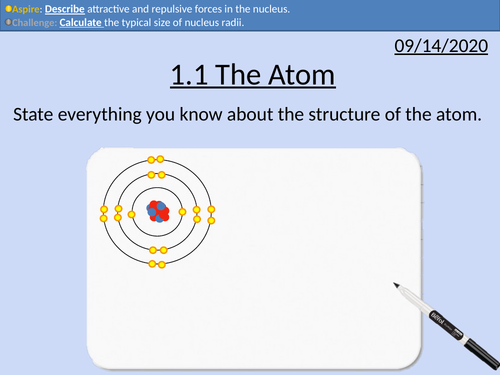
OCR Applied Science: 1.1 The Atom
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.1 of Science Fundementals of the OCR Applied Science Spec.
nucleus contains protons and neutrons surrounded by electrons
relative masses and charges
nuclear and atomic diameters
nucleon number, proton number and isotopes
proton number defines the type of atom
nuclear notation
attractive and repulsive forces within the nucleus

OCR Applied Science: 2.2 Reactions
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.2 of Module 1: Science Fundementals of the OCR Applied Science Spec.
Oxidation and reduction (redox) reactions
Addition reactions of alkenes to include full balanced symbol equations
Substitution reactions of alkanes and haloalkanes to include full balanced
equations
Addition polymerisation to include identification of monomers and repeating units
Condensation polymerisation to include identification of monomers and repeating units
Definition of a radical
The role played by UV light in producing chlorine radicals from CFCs in the
depletion of the ozone layer
Equations to show how chlorine radicals can destroy many ozone molecules
Displacement reactions to include full balanced equations for metals and halogens.

GCSE Chemistry: Purification and Checking Purity
This PowerPoint presentation with worked examples and student questions covers:
• Choosing the correct separation technique
• Comparisons of mobile and stationary phases for chromatography
• Rf Values
• Analysing chromatographs in gas chromatography

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).

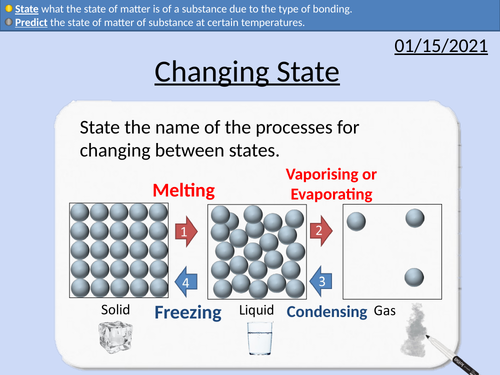
GCSE Chemistry: Changing State
This PowerPoint presentation with worked examples and student questions covers:
• Define melting and boiling point of a pure substance.
• Predict the state of matter of substance at certain temperatures.
• State what the state of matter is of a substance due to the type of bonding.
• Metals, covalent structures, ionic structures and simple molecules.

OCR Applied Science: 21.2.2 Testing During Development
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.2 of Module 21: Product Testing Techniques.
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
Bundle

OCR Applied Science: 21.2 Product Testing of Consumer Products
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
2.3 Effectiveness of test i.e.:
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use (e.g. toxicity, possible mutagenic and
teratogenic effects, microbiological safety)

GCSE Chemistry: Nanoparticles
This PowerPoint presentation with worked examples and student questions covers:
• Relative size of nanoparticles
• Convert nanometres using standard form
• Uses and dangers of nanoparticles

GCSE Chemistry: Formulae of Elements and Molecules
This PowerPoint presentation with worked examples and student questions covers:
• State the number of elements in a chemical formula.
• Determine the chemical formula from display formula.
• Dot and cross diagrams for bonded atoms

OCR Applied Science: 21.2.3 Effectiveness of Tests
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.3 of Module 21: Product Testing Techniques.
2.3 Effectiveness of test
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use

GCSE Chemistry: Conservation of Mass
This PowerPoint presentation with worked examples and student questions covers:
• State the number of atoms from a chemical formula.
• Relative Atomic masses and relative formula mass
• Practical activity of non-closed chemical reactions.
Bundle

GCSE OCR Chemistry C2 Elements, Compounds, and Mixtures
Resources for P2 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Relative Formula Mass
Empirical Formula
Pure and Impure Substances
Filtration and Crystallisation
Simple Distillation
Paper Chromatography
Purification and Checking Purity
Metals and Non-metals
Electronic Structures
Forming Ions
Ionic Compounds
Simple Molecules
Giant Covalent Structures
Polymer Molecules
Structure of Metals
Carbon
Changing State
Bulk Properties
Nanoparticles
Bundle

GCSE OCR Chemistry C2.3 Properties of Materials
Resources for C2.3 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Carbon
Changing State
Bulk Properties
Nanoparticles

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals

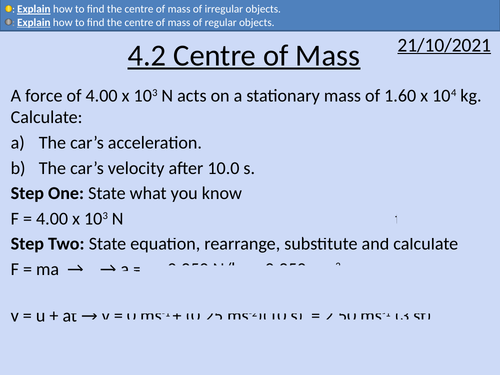
OCR AS level Physics: Centre of Mass
OCR AS level Physics: Centre of mass is a part of the Module 3: Force and Motion
Full lesson PowerPoint with worked examples and homework with complete worked answers.