484Uploads
144k+Views
63k+Downloads
All resources

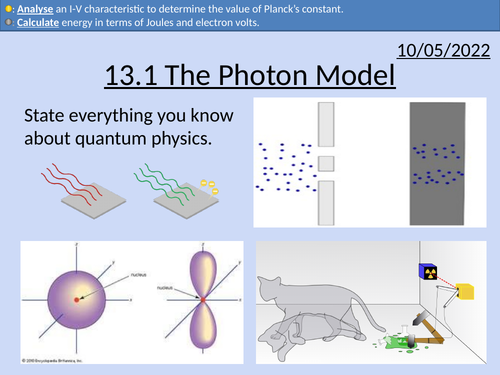
OCR AS Physics: The Photon Model
OCR AS Physics A: The Photon Model is a part of the Module 4: Electrons, Waves, and Photons.
Full lesson PowerPoint with worked examples and homework with complete worked answers.
Energy of a single photon
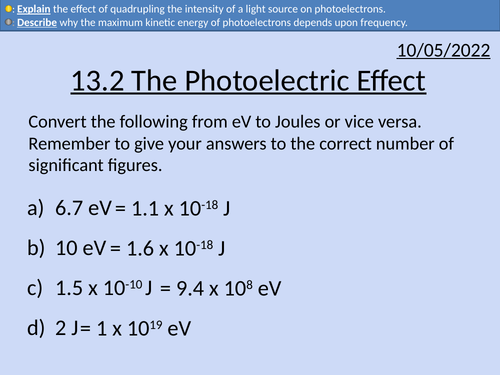
Converting from electron-volts to Joules.
Frequency of the electromagnetic spectrum
Determining Plank’s constant with LEDs
Threshold potential difference difference

GCSE Physics: Energy Resources
This presentation covers OCR Gateway Physics 9-1 P8.2.2 Energy Resources
This PowerPoint is a whole lessons included with student activities and animated answers.
How use of energy resources have changed over time. (Biofuels, Fossil Fuels, Nuclear, Renewable).
How energy use has increased (increase population and development of technology)
Explain patterns and trends in the use of energy resources.
Fossil fuels are finite and will run out at current consumption levels.

OCR AS level Physics: Stopping Distances
OCR AS level Physics: Stopping Distances is a part of the Module 3: Forces and Motion
Presentation come with worked examples, solutions and homeworks.

OCR AS level Physics: Archimedes' Principle
OCR AS level Physics: Archimedes’ Principle is a part of the Module 3: Forces and Motion
Presentation come with worked examples, solutions and homeworks.

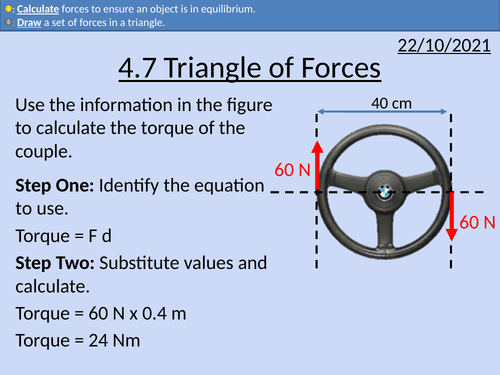
OCR AS level Physics: Triangle of Forces
OCR AS level Physics: Triangle of Forces is a part of the Module 3: Forces and Motion
Presentation come with worked examples, solutions and homeworks.

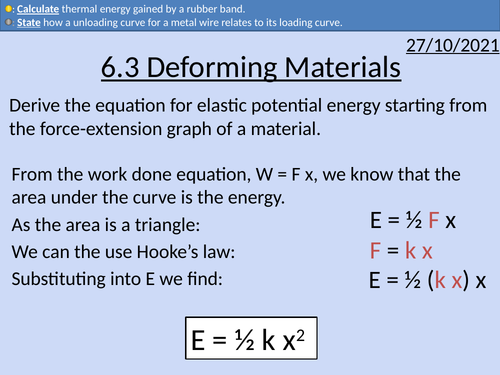
OCR AS level Physics: Deforming Materials
OCR AS level Physics: Deforming Materials is a part of the Module 3: Materials
Presentation come with worked examples, solutions and homeworks.

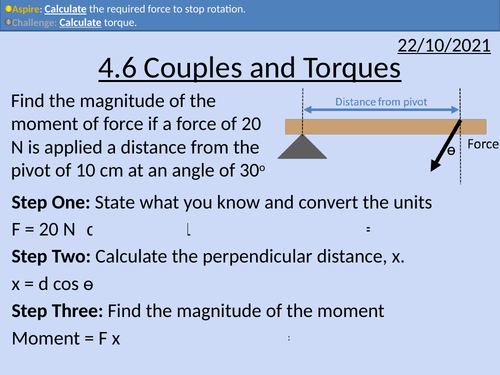
OCR AS level Physics: Couples and Torques
OCR AS level Physics: Couples and Torques is a part of the Module 3: Forces and Motion
Presentation come with worked examples, solutions and homeworks.

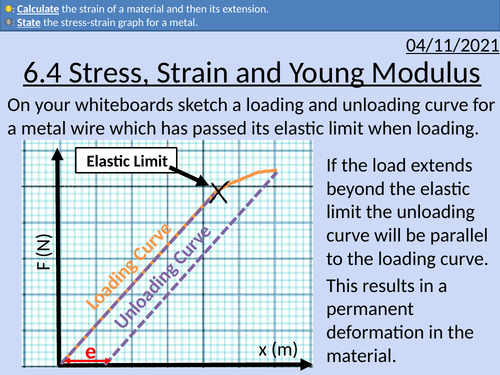
OCR AS level Physics: Stress, Strain, &Young Modulus
OCR AS level Physics: Stress, Strain and Young Modulus is a part of the Module 3: Materials. Presentations come with worked examples, solutions and homeworks.

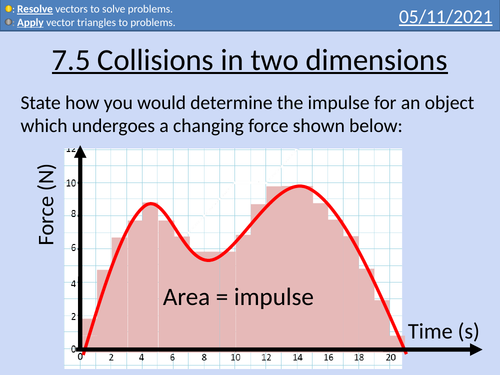
OCR AS level Physics: Collisions in two dimensions
OCR AS level Physics: Collisions in 2D is a part of the Module 3: Laws of Motion and Momentum. Presentations come with worked examples, solutions and homeworks.

OCR AS level Physics: Superposition
OCR AS level Physics: Superposition of Waves is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

OCR AS level Physics: Resistance and Resistivity
OCR AS level Physics: Resistance and Resistivity is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Factors affecting resistance
Calculating resistivity
Resistivity and temperature
Experimentally determining resistivity
Using a graph to calculate resistivity

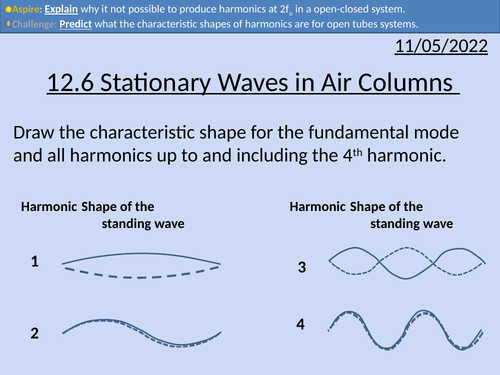
OCR AS level Physics: Stationary Waves in Air Columns
OCR AS level Physics: Stationary Waves in Air Columns is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

OCR AS level Physics: Harmonics
OCR AS level Physics: Harmonics is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

OCR AS level Physics: The Photoelectric Effect
OCR AS level Physics: The Photoelectric Effect is a part of the Module 4: Electrons, Waves, and Photons.
Full lesson PowerPoint with worked examples and homework with complete worked answers.
Threshold frequency
Producing photoelectrons
Kinetic energy of photoelectrons
Linking frequency and wavelength
The electromagnetic spectrum, frequency and energy.

GCSE Chemistry: Development of the Atomic Model
This PowerPoint presentation with worked examples and student questions covers:
• Dalton, Thomson, Rutherford and Bohr’s models
• Comparing different scientific models of the atom

OCR Applied Science: 2.1 Mixtures and Alloys
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.1 of Science Fundementals of the OCR Applied Science Spec.
Types of mixtures to include solutions, colloids and suspensions
Difference between colloids and suspensions in terms of particle size
Uses of common colloids in nature and medicine
Types of colloids to include aerosols, emulsions, foams, gels and sols
Significance of colloids in nature and medicine
Alloys as mixtures of metals
The character and features of alloys
Uses of common alloys to include amalgam, solder, bronze, titanium alloy

GCSE Chemistry: Paper Chromatography & Rf Values
This PowerPoint presentation with worked examples and student questions covers:
• Definition of technique for paper chromatography
• Experimental procedure
• Definitions of stationary and mobile phase
• Application of Rf equation with examples and answers

GCSE Chemistry: Simple Distillation
This PowerPoint presentation with worked examples and student questions covers:
• Changes of state
• The technique of simple distillation
• Concentration of solute increasing in distillation
• Jobs related to chemistry
• Key word test Insoluble, Soluble, Solvent, Solute, Solution, Distillation, Filtration, and Crystallisation

GCSE Chemistry: The pH scale
This PowerPoint presentation with worked examples and student questions covers:
• pH 0 - 14 scale with household examples
• Definitions for acids, bases and alkali substances
• Universal indicator and pH probes
• Using equalities and inequalities

OCR Applied Science: 21.3 Titration Techniques
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
3.1 Titration techniques on consumer products
• Acid-base titration (e.g. limescale removers, eco-disinfectants)
• Precipitation titration (e.g. contact lens saline solution)
• Redox titration, (e.g. bleach, tooth whitener; vitamin C tablets).
• Complexometric titrations (e.g. Milk of Magnesia)
Including explanation and activities on:
Titration calculations
Moles and molar mass
Rearranging Equations
State symbols
Significant Figures
Comparing Data