497Uploads
169k+Views
72k+Downloads
All resources

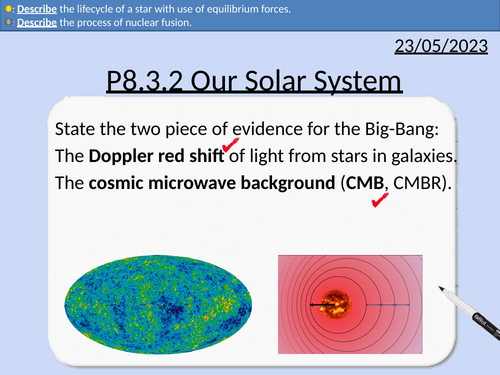
GCSE Physics: The Solar System
This presentation covers OCR Gateway Physics 9-1 P8.3.2 Our Solar System
This PowerPoint is a whole lessons included with student activities and animated answers.
Structure of the solar system
Nuclear Fusion
Evolution of large stars
Evolution of Sun like stars
Gravitational force and force from nuclear fusion

OCR Applied Science: 1.3 Ionic and Covalent Bonding
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.3 of Science Fundementals of the OCR Applied Science Spec.
Elements react together to form compounds by i.e.
ionic bonding
covalent bonding

OCR Applied Science: 6.2 Physico-chemical Properties of Materials
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Structure of metals, giant covalent, and simple molecular structures.
Properties of metals, giant covalent, and simple molecular structures.
Forces and bonds of metals, giant covalent, and simple molecular structures.
Phase diagrams – interpreting and calculating changes.
Sublimation and phase diagrams.

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Covalent Structures
This PowerPoint presentation with worked examples and student questions covers:
• Definition of giant covalent structures
• An empirical formula shows the simplest whole-number ratio of the atoms of each compound.
• Melting and boiling point of simple molecules
• Compare physical properties of simple molecules and giant covalent lattices.

GCSE Chemistry: The pH scale
This PowerPoint presentation with worked examples and student questions covers:
• pH 0 - 14 scale with household examples
• Definitions for acids, bases and alkali substances
• Universal indicator and pH probes
• Using equalities and inequalities

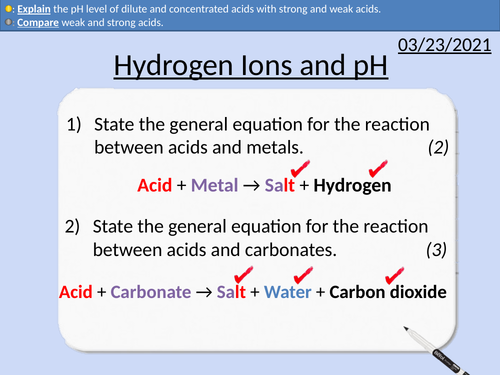
GCSE Chemistry: Hydrogen Ions and pH
This PowerPoint presentation with worked examples and student questions covers:
• Concentration of fruit squash
• Comparing strong and weak acids
• pH and hydrogen ion concentration
• Titration curves

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals

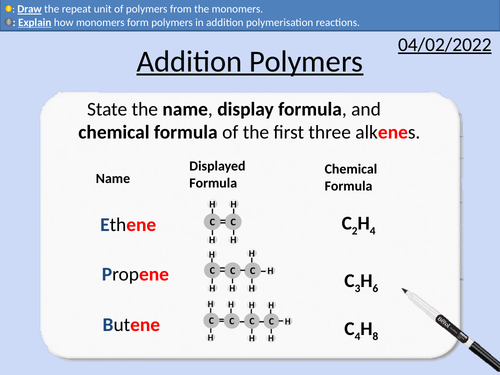
GCSE Chemistry: Addition Polymers
This PowerPoint presentation with worked examples and student questions covers:
Prefixes mono- and poly-
Alkanes and alkenes functional groups
Saturated and unsaturated carbon bonds
Addition polymerisation reactions
Conditions needed for polymerisation reactions
How monomers form polymers
Repeat units and monomers

GCSE Chemistry: Condensation Polymers
This PowerPoint presentation with worked examples and student questions covers:
Block notation for hydrocarbons
Amino acids functional groups
Amino acids forming proteins through condensation reactions
Forming polyesters through condensation reactions
Forming polyamides through condensation reactions
Comparing polyesters and polyamides
Conditions for condensation polymers

OCR AS Chemistry: Introduction to Reaction Mechanisms
OCR AS Chemistry: 11.5 Introduction to Reaction Mechanisms
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Covalent bonds
Homolytic fission and heterolytic reactions
Curly arrows in reaction mechanisms
Identifying addition, substitution, and elimination reactions.

GCSE OCR Physics: Circuit Calculations
This presentation covers OCR Gateway Physics 9-1 P3.2.6 Circuit Calculations.
Measuring current and potential difference
Adding resistors in series and parallel
Rearranging Ohm’s Law
Rules for series circuits
Worked solutions to series circuit exam questions
Rules for parallel circuits
Worked solutions to parallel circuit exam questions

GCSE Physics: Gravitational and Elastic Energy
This presentation covers OCR Gateway Physics 9-1 P7.1.7 Gravitational and Elastic Energy
Energy transfers with links to sport and PE.
Employer-mentor links with physics and sports
Exam Style Question with worked solutions
Rearranging equations
Practice Questions with worked solutions

GCSE Physics: Work Done, Kinetic and Thermal Energy
This presentation covers OCR Gateway Physics 9-1 P7.1.3 Work Done, Kinetic and Thermal Energy
Comes complete with students activities and fully worked solutions.
Energy transfers to thermal store.
Thermal energy, equation, and specific heat capacity.
Work done equation and kinetic energy equation.
Rearranging equations.
Applying equations.
Student questions with fully worked solutions.

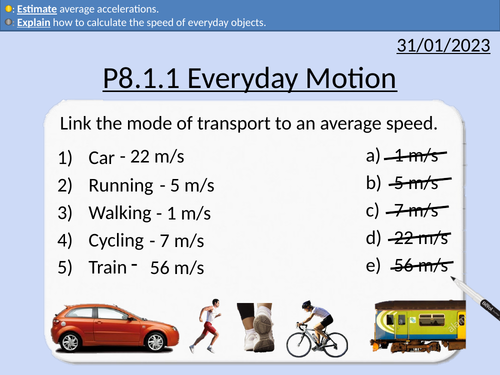
GCSE Physics: Everyday Motion
This presentation covers OCR Gateway Physics 9-1 P8.1.1 Everyday Motion. All presentations come with student activities and worked solutions.
Average speeds of walking, running, cycling, cars, trains, wind, sound, and light.
The speed equation
The acceleration equation
Explaining average speed camera
Explaining instantaneous speed camera
Estimating everyday accelerations
Calculating speed from rotation speed and circumference of wheels
Converting from miles per hour to meters per second

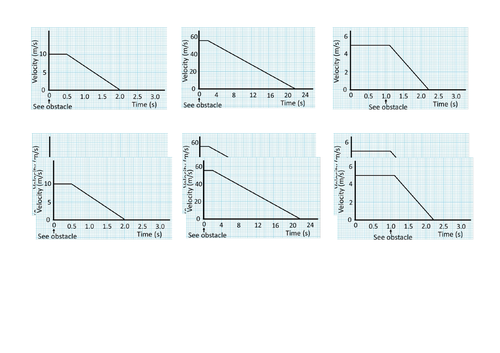
GCSE Physics: Braking and Stopping Distances
This presentation covers OCR Gateway Physics 9-1 P8.1.3 Braking and Stopping Distances. All presentations come with student activities and worked solutions.
Factors affecting braking distance
Total stopping distances
Calculating area of a velocity-time graph for displacement (distance traveled).
Rearranging equations
MOT testing
(Final velocity)2 – (Initial velocity)2 = 2 x Acceleration x Distance
v2 – u2 = 2 a s

GCSE Physics: Current and Forces with Equation
This presentation covers OCR Gateway Physics 9-1 P4.2.1 b Current and Forces.
Units for Magnetic Field Strength
Converting from mT to T
Magnetic Force Equation
Rearranging Equations
Increasing the force on a current carrying conductor in an external magnetic field.
Student questions and worked answers

GCSE Physics: Scientific Defintions and Speed Experiment
This PowerPoint presentation with worked examples and student questions covers:
• Definitions of accurate and precise
• Definition of resolution
• Definition of repeatable and reproducible
• Planning an experiment for determining speed

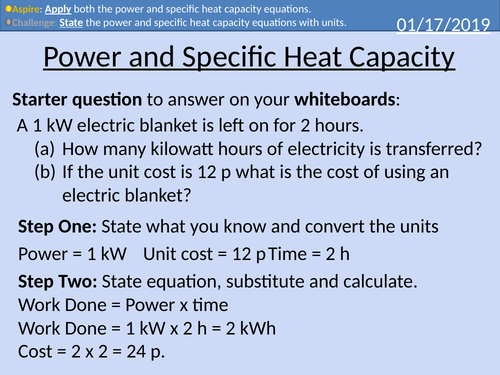
GCSE Physics: Power and Specific Heat Capacity
This presentation covers OCR Gateway Physics 9-1 P7.2.3 Power and Specific Heat Capacity.
• Energy Transfers
• Equations and units
• Worked Exam Style Question
• Student questions with numerical solutions

GCSE Physics: Thermal Conducitvity and Cooling Curves
These two lesson presentations covers OCR Gateway Physics 9-1 P7.2.4 Thermal conductivity and Cooling Curves
Definition for thermal conductivity
Energy transfers and conservation of energy
Reducing energy dissipation
Practical procedure and results analysis