497Uploads
168k+Views
71k+Downloads
Chemistry

GCSE Chemistry: Reaction Profiles
This PowerPoint presentation with worked examples and student questions covers:
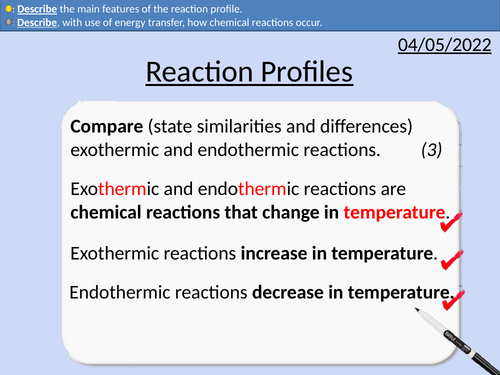
• Reaction profiles for exothermic and endothermic
• Energy stores of particles and surroundings
• Activation energy
• Describing the main features of reaction profiles.

GCSE Chemistry: The pH scale
This PowerPoint presentation with worked examples and student questions covers:
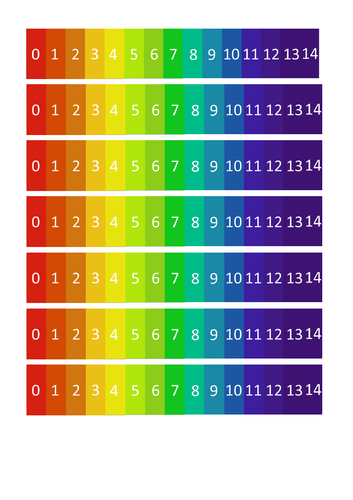
• pH 0 - 14 scale with household examples
• Definitions for acids, bases and alkali substances
• Universal indicator and pH probes
• Using equalities and inequalities

GCSE Chemistry: Neutralisation Reactions
This PowerPoint presentation with worked examples and student questions covers:
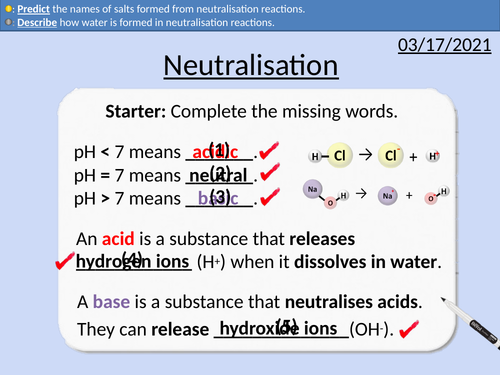
• Word equations for neutralisation reactions
• Describing how ions form salts
• Describing how water is formed
• Predicting the names of salts formed

GCSE Chemistry: Electrolysis of Solutions
This PowerPoint presentation with worked examples and student questions covers:
• The position of metals and non-metals on the periodic table
• The ions metals and non-metals form
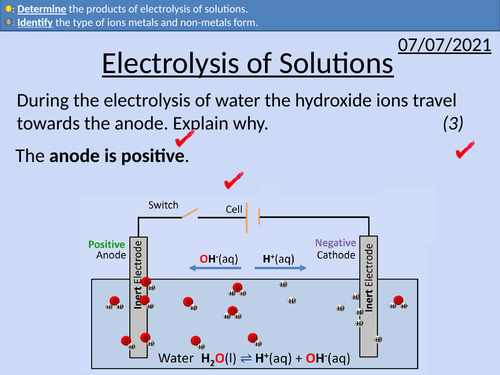
• The ion composition of solutions
• Electrodes, cations and anions
• The products of electrolysis of solutions
• Keyword descriptions and revision tips

GCSE Chemistry: Alkanes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Carbon and hydrogen saturation
• Mnemonic device for naming alkanes
• Comparing complete and incomplete combustion
• Balancing complete combustion reactions

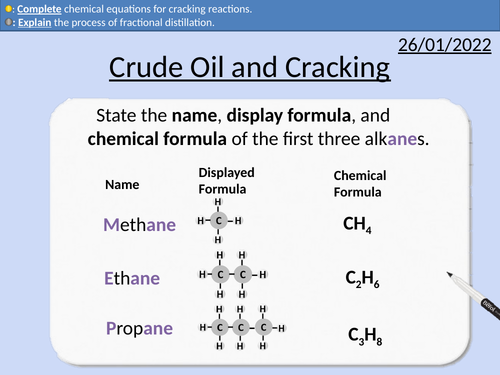
GCSE Chemistry: Crude Oil and Cracking
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Fossil fuels being finite and non-renewable
• Inter-molecular forces and boiling points
• Fractional distillation of crude oil
• Uses of crude oil
• Cracking equations and reasons to crack hydrocarbons

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces

OCR AS Chemistry: Stereoisomerism
OCR AS Chemistry: 13.2 Stereoisomerism
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
E/Z isomerism
Conditions for trans- and cis- isomerism
Cahn-Ingold-Prelog rules and priority ordering

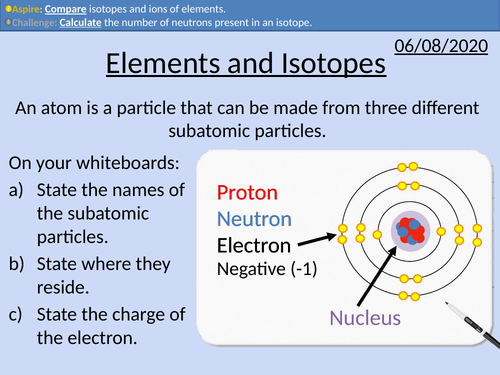
GCSE Chemistry: Isotopes and Ions
This PowerPoint presentation with worked examples and student questions covers:
• Definitions of elements, isotopes, and ions
• State mass number, atomic number, and chemical symbols
• Calculate the number of neutrons
Bundle

GCSE OCR Chemistry: P1.1 The Particle Model
All resources for P1.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Introducing Particles
Chemical and Physical Changes
Limitations of the Particle Model

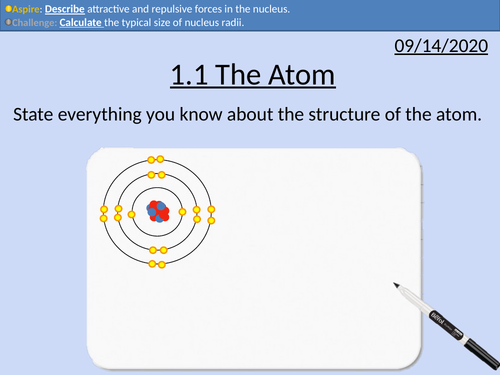
OCR Applied Science: 1.1 The Atom
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.1 of Science Fundementals of the OCR Applied Science Spec.
nucleus contains protons and neutrons surrounded by electrons
relative masses and charges
nuclear and atomic diameters
nucleon number, proton number and isotopes
proton number defines the type of atom
nuclear notation
attractive and repulsive forces within the nucleus

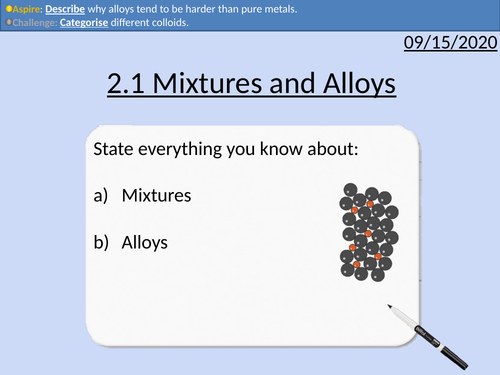
OCR Applied Science: 2.1 Mixtures and Alloys
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.1 of Science Fundementals of the OCR Applied Science Spec.
Types of mixtures to include solutions, colloids and suspensions
Difference between colloids and suspensions in terms of particle size
Uses of common colloids in nature and medicine
Types of colloids to include aerosols, emulsions, foams, gels and sols
Significance of colloids in nature and medicine
Alloys as mixtures of metals
The character and features of alloys
Uses of common alloys to include amalgam, solder, bronze, titanium alloy

GCSE Chemistry: Filtration and Crystallisation
This PowerPoint presentation with worked examples and student questions covers:
Definitions for solution, solute, solvent, insoluble, soluble.
The technique of filtration
The technique of crystallisation

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

GCSE Chemistry: Bond Energies and Energy Changes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of bond energies
• Calculating bond energies per mole
• Calculating change in bond energies in reactions
• Determining if a reaction is exothermic or endothermic from the change in bond energy.

GCSE Chemistry: Exothermic and Endothermic Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition for exothermic and endothermic
• Examples of exothermic and endothermic reactions
• Practical procedure for NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
• Determining if experimental evidence show a exothermic or endothermic reaction

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases
Bundle

GCSE OCR Chemistry C4.1 Predicting and identifying reactions and products
C4.1 Predicting and identifying reactions and products
All resources for P4.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Group 1 - The Alkali Metals
Group 7 - The Halogens
Halogen Displacement Reactions
Group 0 - The Noble Gases
The Transition Metals
Reactivity of Elements

OCR AS Chemistry: Properties of Alkenes
OCR AS Chemistry: 13.1 Properties of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.