496Uploads
162k+Views
70k+Downloads
Chemistry

GCSE Chemistry: Alkenes
This PowerPoint presentation with worked examples and student questions covers:
• Unsaturated hydrocarbons
• Comparing alkanes and alkenes
• Mnemonic device for naming alkenes
• General formula for alkenes
• Completing addition reactions for alkenes

GCSE Chemistry: Alkanes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Carbon and hydrogen saturation
• Mnemonic device for naming alkanes
• Comparing complete and incomplete combustion
• Balancing complete combustion reactions

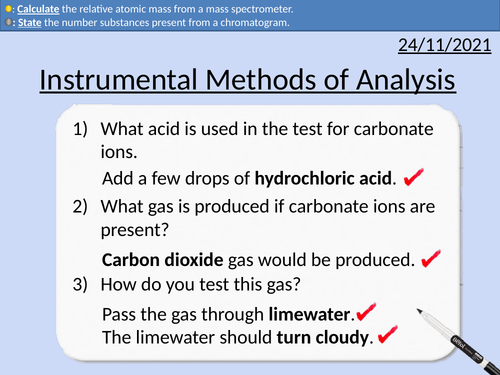
GCSE Chemistry: Instrumental Methods of Analysis
This PowerPoint presentation with worked examples and student questions covers:
Jobs in Environmental Chemistry
Definition of Instrumental Methods of Analysis
Advantages of Instrumental Methods of Analysis
Gas Chromatography and Chromatograms
Mass Spectrometer and Relative Atomic Mass
Identifying a molecule with use of a mass spectrum

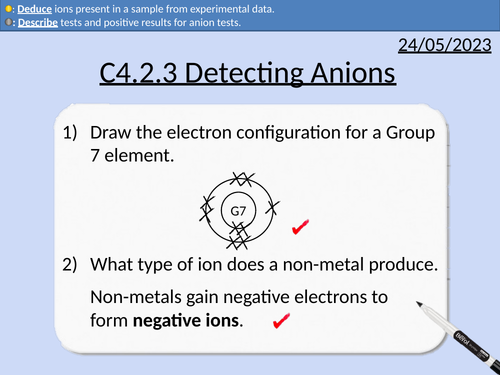
GCSE Chemistry: Detecting Anions
This PowerPoint presentation with worked examples and student questions covers:
Definitions for anions, cations, anodes, cathodes.
Tests for carbonate ions
Tests for sulfate ions
Tests for halide ions

GCSE Chemistry: Detecting Cations
This PowerPoint presentation with worked examples and student questions covers:
Flame tests for lithium, sodium, potassium, calcium, and copper.
Electron energy levels and emitting radiation.
Precipitate tests for iron(II)), iron(III), copper(II), calcium, and zinc.

GCSE Chemistry: Detecting Gases
This PowerPoint presentation with worked examples and student questions covers:
Tests for Hydrogen, Oxygen, Carbon Dioxide, Chlorine.
Gifs of each gas test
Electron structure for diatomic molecules
Bundle

GCSE OCR Chemistry C4.1 Predicting and identifying reactions and products
C4.1 Predicting and identifying reactions and products
All resources for P4.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Group 1 - The Alkali Metals
Group 7 - The Halogens
Halogen Displacement Reactions
Group 0 - The Noble Gases
The Transition Metals
Reactivity of Elements

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals

GCSE Chemistry: Transition Metals
This PowerPoint presentation with worked examples and student questions covers:
• Properties of transition metals gases
• Comparing transition metals with alkali metals
• Everyday applications of transition metals
• Transition metals as catalysts

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases

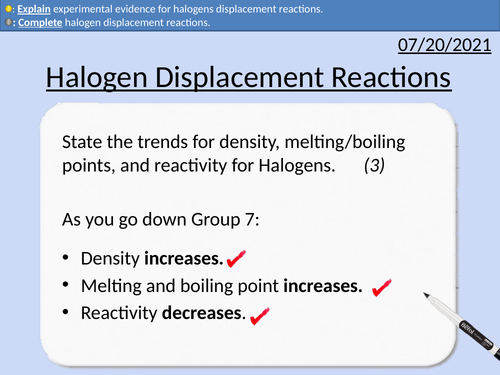
GCSE Chemistry: Halogen Displacement Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition of halides displacement reactions
• Definition of displacement reactions
• Identifying displaced products
• Completing displacement reactions
• Explaining experimental evidence for displacement reactions.

GCSE Chemistry: Group 7 - Halogens
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Halogens
• Trends and anomalies in Group 7 (Density, Melting Point)
• Reactivity of Group 7 Halogens
• Electron configuration of Group 7 Halogens
• Forming salts with alkali metals and halogens

GCSE Chemistry: Group 1 - Alkali Metals
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Alkali Metals
• Trends and anomalies in Group 1 (Density, Melting Point)
• Reactivity of Group 1 Alkali Metals
��� Electron configuration of Group 1 Alkali Metals

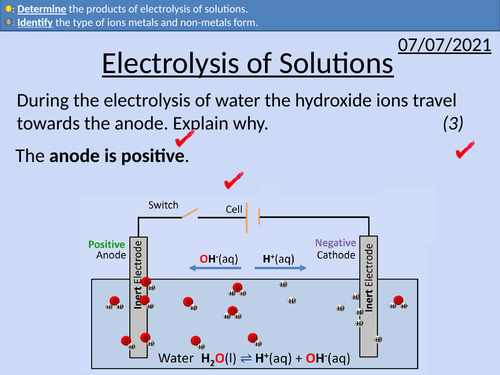
GCSE Chemistry: Electrolysis of Solutions
This PowerPoint presentation with worked examples and student questions covers:
• The position of metals and non-metals on the periodic table
• The ions metals and non-metals form
• The ion composition of solutions
• Electrodes, cations and anions
• The products of electrolysis of solutions
• Keyword descriptions and revision tips

GCSE Chemistry: Electrolysis of Water
This PowerPoint presentation with worked examples and student questions covers:
• Pure water being made partially of ions (hydrogen and hydroxide).
• PANIC convention for electrodes
• OILRIG convention for redox reactions
• Electron transfers at electrodes
• Half-equations for anode and cathode
• Balancing half-equations

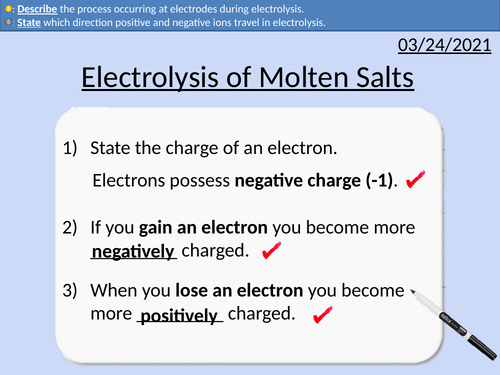
GCSE Chemistry: Electrolysis of molten salts
This PowerPoint presentation with worked examples and student questions covers:
• Naming electrolysis experimental set up
• PANIC convention for electrodes
• Electron transfers at electrodes
• Half-equations for anode and cathode

GCSE Chemistry: Hydrogen Ions and pH
This PowerPoint presentation with worked examples and student questions covers:
• Concentration of fruit squash
• Comparing strong and weak acids
• pH and hydrogen ion concentration
• Titration curves

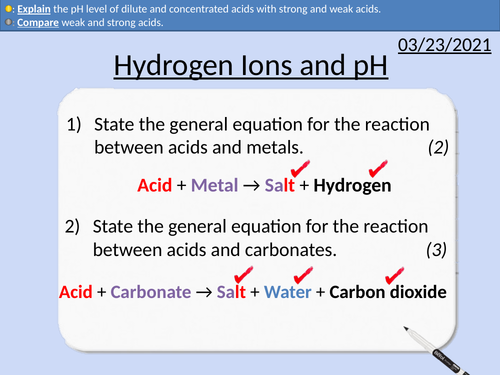
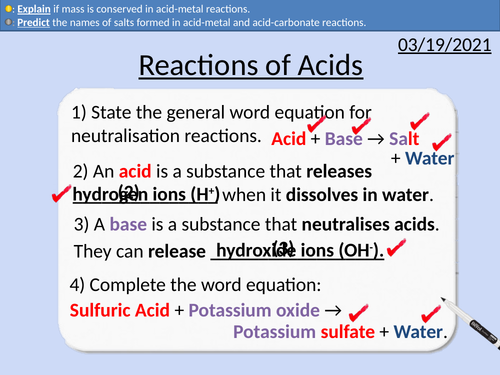
GCSE Chemistry: Reactions of Acids
This PowerPoint presentation with worked examples and student questions covers:
• Identifying metals on the periodic table
• Predicting the salt formed in acid metal reactions.
• Predicting the salt formed in acid carbonate reactions.
• Conservation of mass and state symbols

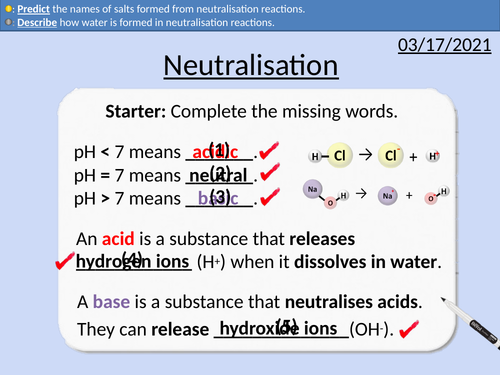
GCSE Chemistry: Neutralisation Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Word equations for neutralisation reactions
• Describing how ions form salts
• Describing how water is formed
• Predicting the names of salts formed

GCSE Chemistry: The pH scale
This PowerPoint presentation with worked examples and student questions covers:
• pH 0 - 14 scale with household examples
• Definitions for acids, bases and alkali substances
• Universal indicator and pH probes
• Using equalities and inequalities