496Uploads
162k+Views
70k+Downloads
Chemistry

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces

OCR AS Chemistry: Properties of Alcohols
OCR AS Chemistry: 14,1 Properties of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces

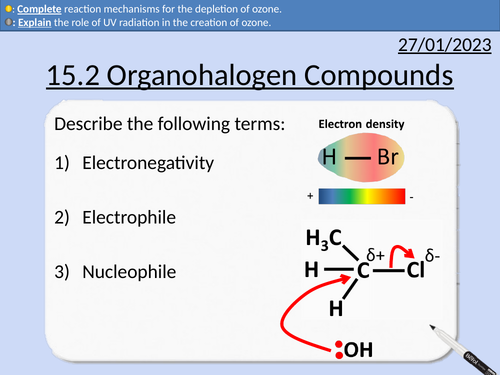
OCR AS Chemistry: Organohalogen Compounds
OCR AS Chemistry: 15.2 Organohalogen Compounds and the Environment
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definitions for CFC (Chlorofluorocarbons) and HCFC (Hydachlorofluorocarbons)
Creation of ozone
Depletion of ozone with CFCs
Reaction steps including initiations and propagation

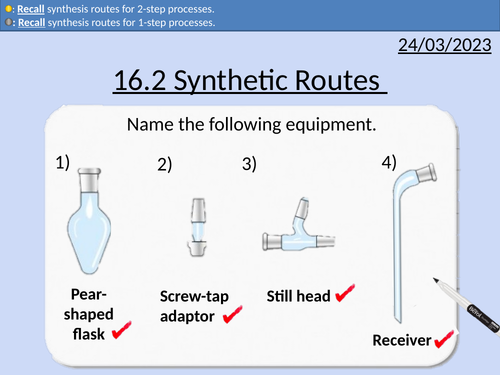
OCR AS Chemistry: Synthetic Routes
OCR AS Chemistry: 16.2 Synthetic Routes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Functional Groups - Alkane, Alkene, Haloalkane, Alcohols, Carboxylic Acid, Ketone, Aldehyde, Ester, Amine, Nitrile.
One-step synthetic routes with reagents and conditions
Two-step synthetic routes with reagents and conditions

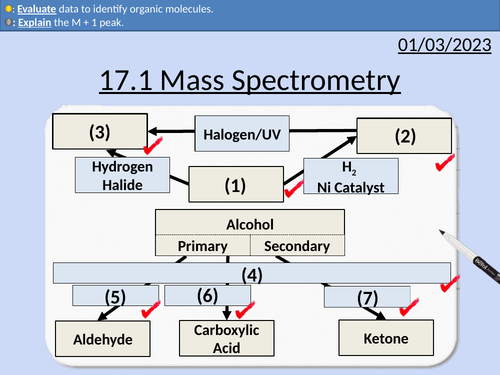
OCR AS Chemistry: 17.1 Mass Spectrometry
OCR AS Chemistry: 17.1 Mass Spectrometry
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Molecular ions M+
M + 1 peak
Fragment ions
Identifying molecules from a mass spectrum

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

GCSE Chemistry: Bond Energies and Energy Changes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of bond energies
• Calculating bond energies per mole
• Calculating change in bond energies in reactions
• Determining if a reaction is exothermic or endothermic from the change in bond energy.

GCSE Chemistry: Group 7 - Halogens
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Halogens
• Trends and anomalies in Group 7 (Density, Melting Point)
• Reactivity of Group 7 Halogens
• Electron configuration of Group 7 Halogens
• Forming salts with alkali metals and halogens

GCSE Chemistry: Detecting Cations
This PowerPoint presentation with worked examples and student questions covers:
Flame tests for lithium, sodium, potassium, calcium, and copper.
Electron energy levels and emitting radiation.
Precipitate tests for iron(II)), iron(III), copper(II), calcium, and zinc.

GCSE Chemistry: Alkenes
This PowerPoint presentation with worked examples and student questions covers:
• Unsaturated hydrocarbons
• Comparing alkanes and alkenes
• Mnemonic device for naming alkenes
• General formula for alkenes
• Completing addition reactions for alkenes

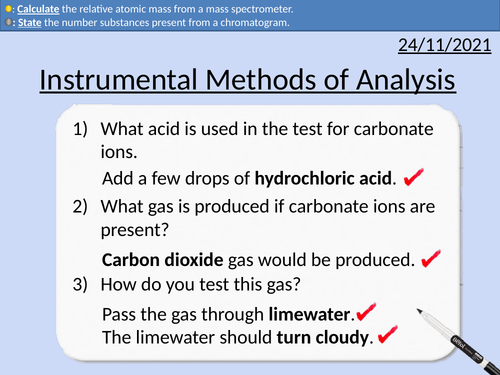
GCSE Chemistry: Instrumental Methods of Analysis
This PowerPoint presentation with worked examples and student questions covers:
Jobs in Environmental Chemistry
Definition of Instrumental Methods of Analysis
Advantages of Instrumental Methods of Analysis
Gas Chromatography and Chromatograms
Mass Spectrometer and Relative Atomic Mass
Identifying a molecule with use of a mass spectrum

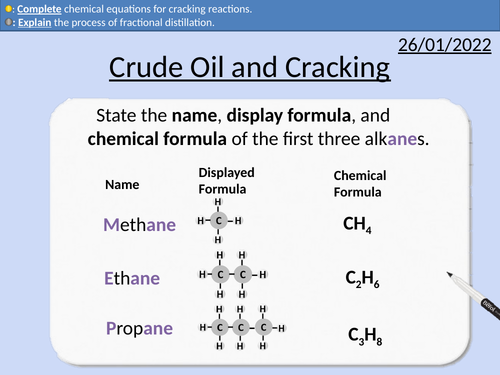
GCSE Chemistry: Crude Oil and Cracking
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Fossil fuels being finite and non-renewable
• Inter-molecular forces and boiling points
• Fractional distillation of crude oil
• Uses of crude oil
• Cracking equations and reasons to crack hydrocarbons

OCR AS Chemistry: Organic Chemistry
OCR AS Chemistry: 11.1 Organic Chemistry
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definition of hydrocarbons
What organic chemistry is
Saturated and unsaturated hydrocarbons
Definition of functional groups
Definition of homologous group

OCR AS Chemistry: Reactions of Alkenes
OCR AS Chemistry: 13.3 Reactions of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Alkene addition reactions:
Hydrogen with a nickel catalyst
Halogens
Hydrogen halide
Steam with an acid catalyst
Test for unsaturated alkenes.
Bond enthalpy for sigma and pi bonds.

OCR AS Chemistry: Stereoisomerism
OCR AS Chemistry: 13.2 Stereoisomerism
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
E/Z isomerism
Conditions for trans- and cis- isomerism
Cahn-Ingold-Prelog rules and priority ordering

OCR AS Chemistry: Properties of Alkenes
OCR AS Chemistry: 13.1 Properties of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.

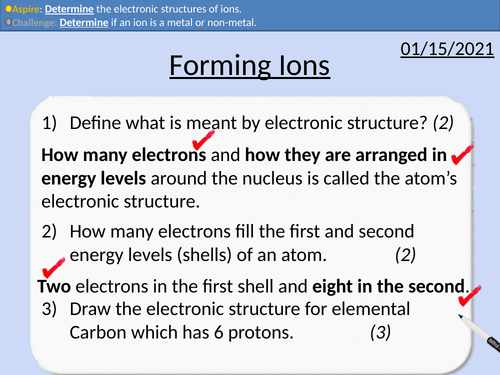
GCSE Chemistry: Forming Ions
This PowerPoint presentation with worked examples and student questions covers:
• Definition of ions
• The electronic configuration of ions
• Ions metals and nonmetals form
• Drawing electron configurations

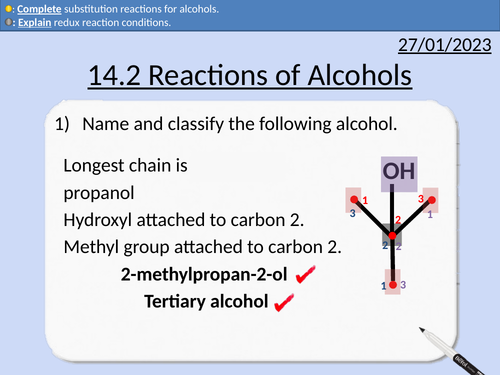
OCR AS Chemistry: Reactions of Alcohols
OCR AS Chemistry: 14.2 Reactions of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Combustion of alcohols
Reflux condition for reactions
Primary alcohol to aldehydes
Primary alcohols to carboxylic acids
Secondary alcohols to ketones
Dehydration of alcohols
Substitution reactions for alcohols

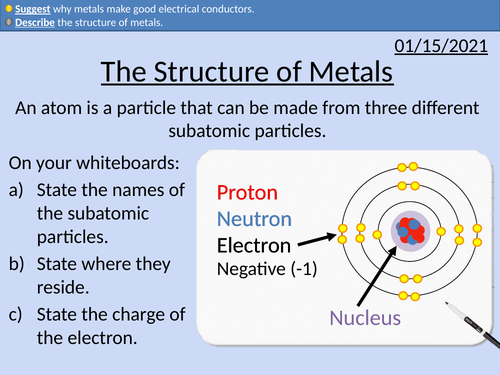
GCSE Chemistry: The Structure of Metals
This PowerPoint presentation with worked examples and student questions covers:
• State a use for metals
• Describe the structure of metals
• Why metals make good electrical conductors.
• Metals on the periodic table

GCSE Chemistry: Electrolysis of Water
This PowerPoint presentation with worked examples and student questions covers:
• Pure water being made partially of ions (hydrogen and hydroxide).
• PANIC convention for electrodes
• OILRIG convention for redox reactions
• Electron transfers at electrodes
• Half-equations for anode and cathode
• Balancing half-equations