484Uploads
146k+Views
64k+Downloads
All resources

OCR Applied Science: 1.2 The Periodic Table
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.2 of Science Fundementals of the OCR Applied Science Spec.
Elements are based on atomic structure and can be classified by the Periodic Table i.e.:
organisation of elements within the table
groups
periods
atomic number
atomic mass atomic radius

GCSE Chemistry: Filtration and Crystallisation
This PowerPoint presentation with worked examples and student questions covers:
Definitions for solution, solute, solvent, insoluble, soluble.
The technique of filtration
The technique of crystallisation

OCR Applied Science: 6.2 Physico-chemical Properties of Materials
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Structure of metals, giant covalent, and simple molecular structures.
Properties of metals, giant covalent, and simple molecular structures.
Forces and bonds of metals, giant covalent, and simple molecular structures.
Phase diagrams – interpreting and calculating changes.
Sublimation and phase diagrams.

OCR Applied Science: 6.3 Electrical Properties
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.3 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Current as flow of charge in a conductor.
Use the equation: I = ΔQ ÷ Δt
Ohm’s law illustrates the relationship of V ∝ I
Use the equation: potential difference (V) = current (A) × resistance
Use the equations for adding resistors in series and parallel
Compare electromotive force and potential difference
Use the equation: charge © = current (A) × time (s)
Use and recognise the equation for mean drift velocity
Use the equation: energy transferred (work done) (J) = charge © × potential difference (V)
Use the equation: energy transferred (J, kWh) = power (W, kW) × time (s, h)
Use the equation: power (W) = energy (J) ÷ time (s)

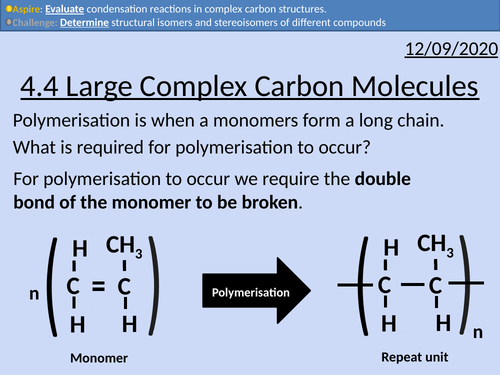
OCR Applied Science: 4.4 Large Complex Carbon Molecules
This PowerPoint presentation with worked examples and student activities covers: Topic 4.4 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Complex carbohydrates (starch, glycogen, cellulose)
• Carbohydrates found as monosaccharides, disaccharides, or polysaccharides (monomers, dimers or polymers)
• Monomers held together by glycosidic bonds to form dimers and polymers, via condensation reactions
• Monosaccharides include glucose, fructose and galactose
• Disaccharides include maltose, sucrose and lactose
• Polysaccharides include starch, glycogen and cellulose
• Cellulose is found in plant cell walls where it provides strength/support and pliability
• Starch and glycogen are energy sources
Proteins and peptides from amino acids
• Dipeptides are formed from two amino acids joined by a peptide bond, via a condensation reaction
• Polypeptides are chains of amino acids joined by peptide bonds
• Proteins/polypeptides have physiological or functional roles, including enzymes, carrier proteins in the plasma membrane, and structural roles, including collagen and elastin fibres in connective tissue
Lipids from fatty acids, glycerol and phosphorus compounds
• Monoglycerides, diglycerides and triglycerides are esters of fatty acids and glycerol
• An ester bond forms between each fatty acid and the glycerol, via condensation reactions
• Phospholipids contain glycerol plus two fatty acids and a phosphate group
• Lipids act as an energy source within cells, as an insulation layer around animal organs, in the myelin sheath (found around some nerve fibres/axons) to increase speed of nerve transmission
• Phospholipids form a bilayer in the plasma membrane
Protein synthesis (transcription, translation) RNA, messenger, ribosomal and transfer
• The nucleic acids, DNA and RNA, are polymers of nucleotides
• Peptide bonds form between amino acids to create polypeptide chains/proteins
• Recall a simple description of protein synthesis

GCSE Chemistry: Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• Filled outer shells result in more stable electronic structures.
• The electronic configuration ionic compounds
• Models of giant ionic structures

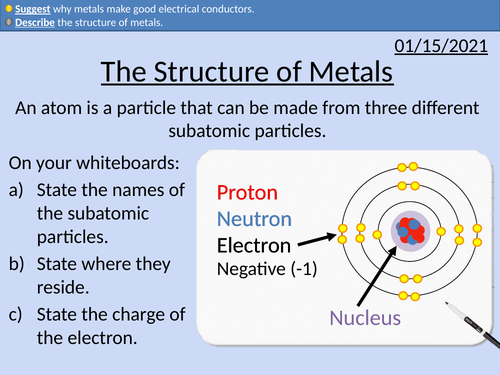
GCSE Chemistry: The Structure of Metals
This PowerPoint presentation with worked examples and student questions covers:
• State a use for metals
• Describe the structure of metals
• Why metals make good electrical conductors.
• Metals on the periodic table

OCR Applied Science: 21.2.1 Types of Testing
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.1 of Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation

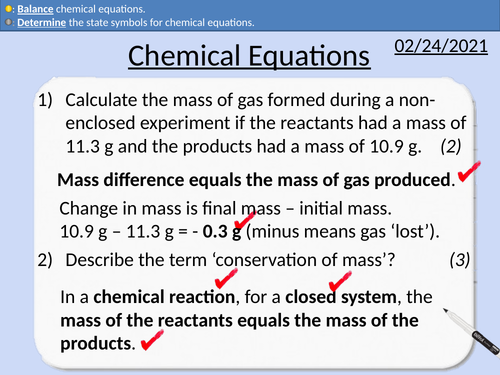
GCSE Chemistry: Chemical Equations
This PowerPoint presentation with worked examples and student questions covers:
• Pathways into medical chemistry
• State the number of atoms from a chemical formula.
• Properties of metals and non-metals
• Determine state symbols for chemical equations
• Balancing chemical equations

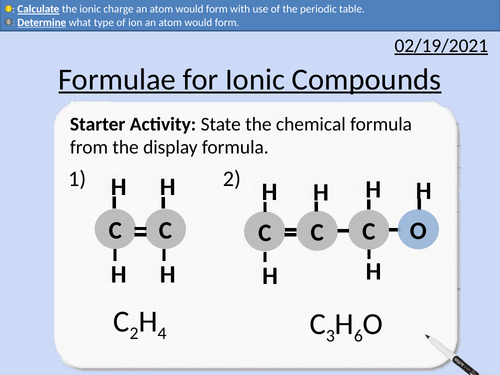
GCSE Chemistry: Formulae for Ionic Compounds
This PowerPoint presentation with worked examples and student questions covers:
• State the number of electrons in each energy level.
• Determine what type of ion an atom would form.
• Calculate the ionic charge an atom would form with use of the periodic table.
• Groups number, outer shell electrons, dot and cross diagrams

GCSE Chemistry: Detecting Gases
This PowerPoint presentation with worked examples and student questions covers:
Tests for Hydrogen, Oxygen, Carbon Dioxide, Chlorine.
Gifs of each gas test
Electron structure for diatomic molecules

GCSE Chemistry: Producing Electricity Using Chemistry
This PowerPoint presentation with worked examples and student questions covers:
Chemical cells uses
Fuel cell uses
Comparing fuel cells and chemical cells
Environmental impact of fuel cells and chemical cells
The structure of fuel cells
The operation of fuel cells
Half-equations for fuel cells

GCSE Physics: Electromagnetic Refraction
This presentation covers OCR Gateway Physics 9-1 P5.3.2a Electromagnetic Reflection.
Includes student activities and full worked answers.
Refraction the change of velocity - speed and direction
Magnitude of refraction depending on wavelength
Magnitude of refraction depending on optical density
Refraction practical activity instructions
Wave speed, wavelength, and frequency relationship in refraction

OCR AS Chemistry: Stereoisomerism
OCR AS Chemistry: 13.2 Stereoisomerism
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
E/Z isomerism
Conditions for trans- and cis- isomerism
Cahn-Ingold-Prelog rules and priority ordering

OCR AS Physics: Diodes
OCR AS Physics A: Diodes is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Polarity of diodes
Conventional current and diodes
Plotting I-V curves for diodes
Describing I-V curves for diodes

OCR AS Physics: Electrical Energy & Power
OCR AS Physics: Electrical Energy & Power is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Derive three equations for electrical power
Applying electrical power equations
Create a circuit diagram to calculate power
Base units for V A and W.

OCR AS Physics: Internal Resistance
OCR AS Physics: Internal Resistance is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Find lost volts and terminal potential difference from a source of emf
Calculate internal resistance of a cell
Calculate internal resistance of a cell with a graph
Calculate EMF of a source

OCR AS Physics: Wave Properties
OCR AS Physics: Wave Properties is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.

GCSE Physics: Electrical Current
This presentation covers OCR Gateway Physics 9-1 P3.1.2 Electrical Current
Conditions for current to flow
Conventional current and electron flow
Measuring current with ammeters
Current at junctions
Converting from mA to A
Rearranging equations
Determining current and charge flow with equation

GCSE Physics: Transformers
This lesson presentations covers OCR Gateway Physics 9-1 P4.2.5 Transformers.
Examples of transformers in everyday life
Structure of a transformer Step-up and Step-down transformers
Transformer equation and rearranging equations.
Worked Examples and Student problems with worked solutions.
Explanation for use of step-up transformers - efficiency