484Uploads
146k+Views
64k+Downloads
All resources

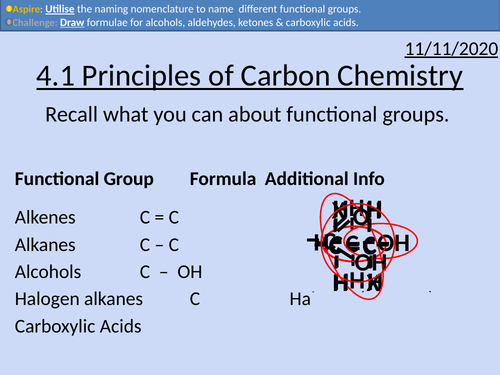
OCR Applied Science: 4.1 Principles of Carbon Chemistry
This PowerPoint presentation with worked examples and student activities covers:
Topic 4.1 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
• Alkanes as saturated hydrocarbons containing single C-C and C-H bonds
• Alkenes as unsaturated hydrocarbons containing a C=C double bond
• Alkynes as unsaturated hydrocarbons containing a C ≡ C triple bond
• Name and draw structural and skeletal formulae of the first four members of alkanes, alkenes and alkynes
• Aldehydes and ketones as organic compounds containing the C=O group
• Name and draw the structural formulae of the first four aldehydes and the first two ketones
• Alcohols as organic compounds containing the OH group
• Name and draw structural and skeletal formulae of the first four alcohols
• Conversion of alcohols to form aldehydes and ketones is classified as an oxidation reaction
• Name and draw structural and skeletal formulae of the first four carboxylic acids
• Reaction of carboxylic acids with an alkali, to include full equations using structural formulae
• Name and draw structural and skeletal formulae of the four C4H8O2 esters
• How an ester can be made from a carboxylic acid and an alcohol

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).

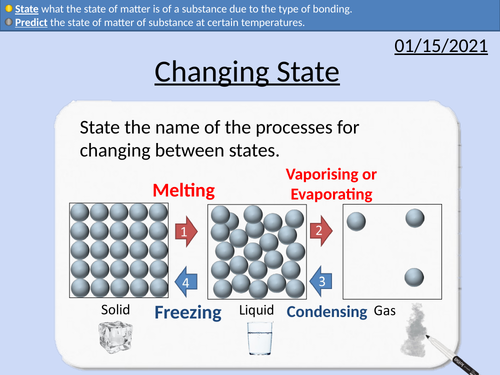
GCSE Chemistry: Changing State
This PowerPoint presentation with worked examples and student questions covers:
• Define melting and boiling point of a pure substance.
• Predict the state of matter of substance at certain temperatures.
• State what the state of matter is of a substance due to the type of bonding.
• Metals, covalent structures, ionic structures and simple molecules.

OCR Applied Science: 21.2.2 Testing During Development
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.2 of Module 21: Product Testing Techniques.
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
Bundle

OCR Applied Science: 21.2 Product Testing of Consumer Products
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
2.1 Types of testing i.e.:
• in-vitro
• in-vivo
• titration
• extraction and separation
2.2 Laboratory testing during development i.e.:
• formulation
• production
• quality control and assurance
• after sale monitoring.
2.3 Effectiveness of test i.e.:
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use (e.g. toxicity, possible mutagenic and
teratogenic effects, microbiological safety)

OCR Applied Science: 21.2.3 Effectiveness of Tests
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
This PowerPoint presentation with worked examples and student activities covers: Topic 2.3 of Module 21: Product Testing Techniques.
2.3 Effectiveness of test
• Appropriate test method
• Data collection validity and reliability
• Consistent chemical composition
• Hazards and risks of use

GCSE Chemistry: Conservation of Mass
This PowerPoint presentation with worked examples and student questions covers:
• State the number of atoms from a chemical formula.
• Relative Atomic masses and relative formula mass
• Practical activity of non-closed chemical reactions.
Bundle

GCSE OCR Chemistry C2.3 Properties of Materials
Resources for C2.3 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Carbon
Changing State
Bulk Properties
Nanoparticles

GCSE Chemistry: Group 1 - Alkali Metals
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Alkali Metals
• Trends and anomalies in Group 1 (Density, Melting Point)
• Reactivity of Group 1 Alkali Metals
• Electron configuration of Group 1 Alkali Metals

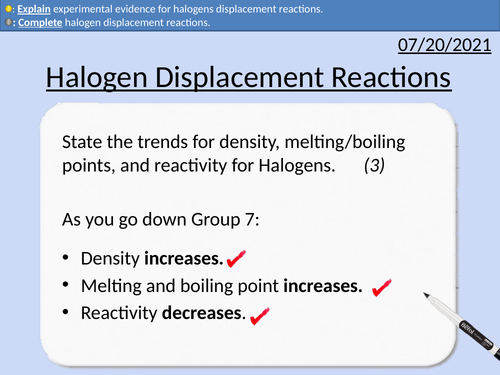
GCSE Chemistry: Halogen Displacement Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition of halides displacement reactions
• Definition of displacement reactions
• Identifying displaced products
• Completing displacement reactions
• Explaining experimental evidence for displacement reactions.

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals

OCR A level Physics: Angular Acceleration
OCR A level Physics: Angular Acceleration and the Radian is a part of the Module 5: Newtonian world and astrophysics.
All presentations come with worked examples, solutions and homeworks with answers.

OCR AS Chemistry: Nomenclature of Organic Compounds
OCR AS Chemistry: 11.2 Nomenclature of Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Aliphatic, alicyclic, and aromatic compounds.
Naming organic compounds
Drawing organic compounds
Functional Groups
Alkane
Alkene
Alkyne
Alcohols
Haloalkane
Aldehyde
Ketone
Carboxylic Acid
Ester
Amine
Nitrile

OCR AS Chemistry: Representing the formulae of Organic Compounds
OCR AS Chemistry: Formulae for Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
molecular formula
empirical formula
general formula
displayed formula
structural formula
skeletal formula
Bundle

GCSE OCR Physics P5.1 Wave Behaviour
Resources for P5.1 GCSE OCR Physics Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Each lesson includes student activities and full worked answers.
Includes:
Definition of a wave
Mechanical waves
Electromagnetic waves
Transverse waves
Longitudinal waves
Amplitude
Wavelength
Frequency
Time period
Calculating frequency and equation
Relationship between frequency and wavelength when speed is constant.
Calculating time period from frequency with equations
The speed equation
Measuring distance and time
Simple experiment for the speed of sound
Improving experiments
Echoes
Speed of sound experiment with microphones and oscilloscope.
Ray diagrams
Absorption, reflection and transmission
Sonar
Ultrasound
Rearranging equation
Refraction
Relationship between wave speed and wavelength
Structure of the ear.
Frequency range of human hearing.
Explanation of the limited frequency range of humans.
Explanation for hearing deteriorating with age.

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces
Bundle

OCR AS level Chemistry: Alkanes
OCR AS level Chemistry: Alkanes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Bundle

GCSE OCR Physics P5.2 Electromagnetic Spectrum
Resources for P5.2 GCSE OCR Physics Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Each lesson includes student activities and full worked answers.
Order of the electromagnetic spectrum
Wavelength and frequency relationship
Application of wave speed equation
Rearranging equation
Producing and detecting radio waves
Recall that light is an electromagnetic wave
Give examples of some practical uses of electromagnetic waves in the radio, micro-wave, infra-red, visible, ultraviolet, X-ray and gamma-ray regions
Describe how ultra-violet waves, X-rays and gamma rays can have hazardous effects, notably on human bodily tissues.
Explain that electromagnetic waves transfer energy from source to absorber to include examples from a range of electromagnetic waves
Precautions for ultra-violet waves, X-rays and gamma rays
Careers: Medical Physicist
X-rays
CT scans
Gamma imaging
Thermogram
Magnetic Resonance Imaging
Precautions for using ionising radiation