496Uploads
163k+Views
70k+Downloads
Chemistry
Bundle

GCSE OCR Chemistry C1 Particles
All resources for P1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
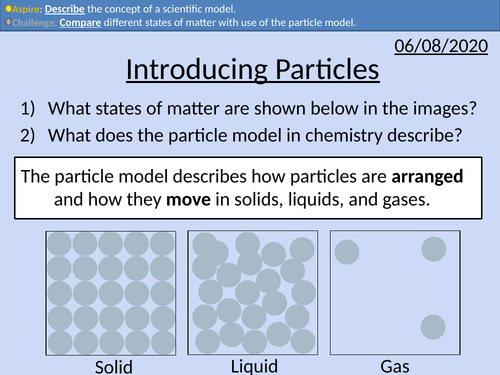
Introducing Particles
Chemical and Physical Changes
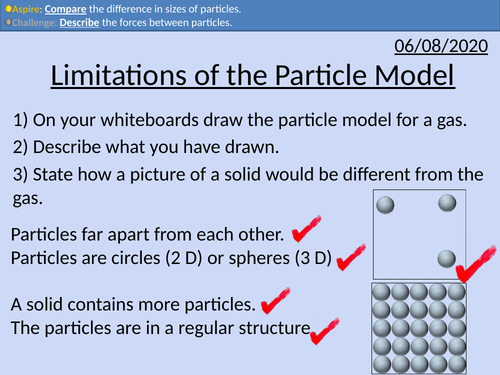
Limitations of the Particle Model
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

OCR AS Chemistry: Chemical Reactions of Alkanes
OCR AS Chemistry: 12.2 Chemical Reactions of Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Combustion reactions
Incomplete combustion reactions
Balancing equations
Using algebraic equations
Radical substitution reactions
Reaction mechanism for haloalkanes - Initiation, Propagation, and Termination
Monosubstituted (positional isomers) isomers

OCR AS Chemistry: Electrophilic Addition in Alkenes
OCR AS Chemistry: 13.4 Electrophilic Addition in Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule

GCSE Chemistry: Limitations of the Particle Model
This PowerPoint presentation with worked examples and student questions covers:
• Describing the limitations of the model: lack of forces between particles, size of particles, and space between the particles.
• Mathematically comparing sizes and distances of particles

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases

OCR AS Chemistry: Representing the formulae of Organic Compounds
OCR AS Chemistry: Formulae for Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
molecular formula
empirical formula
general formula
displayed formula
structural formula
skeletal formula

OCR AS Chemistry: Properties of Alcohols
OCR AS Chemistry: 14,1 Properties of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces

GCSE Chemistry: Introducing Particles
This PowerPoint presentation with worked examples and student questions covers:
• Solids, liquids, and gases
• Scientific models as a concept
Bundle

GCSE OCR Chemistry: P1.2 Atomic Structure
All resources for P1.2 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

OCR Applied Science: 2.2 Reactions
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.2 of Module 1: Science Fundementals of the OCR Applied Science Spec.
Oxidation and reduction (redox) reactions
Addition reactions of alkenes to include full balanced symbol equations
Substitution reactions of alkanes and haloalkanes to include full balanced
equations
Addition polymerisation to include identification of monomers and repeating units
Condensation polymerisation to include identification of monomers and repeating units
Definition of a radical
The role played by UV light in producing chlorine radicals from CFCs in the
depletion of the ozone layer
Equations to show how chlorine radicals can destroy many ozone molecules
Displacement reactions to include full balanced equations for metals and halogens.

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).

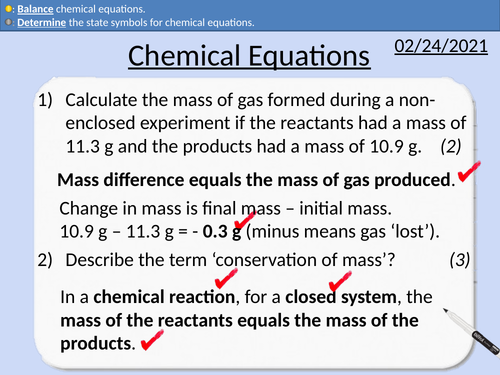
GCSE Chemistry: Chemical Equations
This PowerPoint presentation with worked examples and student questions covers:
• Pathways into medical chemistry
• State the number of atoms from a chemical formula.
• Properties of metals and non-metals
• Determine state symbols for chemical equations
• Balancing chemical equations

GCSE Chemistry: Alkenes
This PowerPoint presentation with worked examples and student questions covers:
• Unsaturated hydrocarbons
• Comparing alkanes and alkenes
• Mnemonic device for naming alkenes
• General formula for alkenes
• Completing addition reactions for alkenes

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces

OCR AS Chemistry: Reactions of Alkenes
OCR AS Chemistry: 13.3 Reactions of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Alkene addition reactions:
Hydrogen with a nickel catalyst
Halogens
Hydrogen halide
Steam with an acid catalyst
Test for unsaturated alkenes.
Bond enthalpy for sigma and pi bonds.

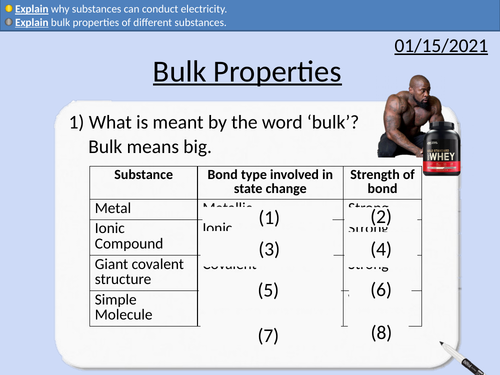
GCSE Chemistry: Bulk Properties
This PowerPoint presentation with worked examples and student questions covers:
• Jobs in Material Science
• Bulk properties of metals - malleable and conductors of electricity
• Bulk properties of ionic and covalent structures - brittle
• Explain why substances conducting electricity depends upon the state of matter

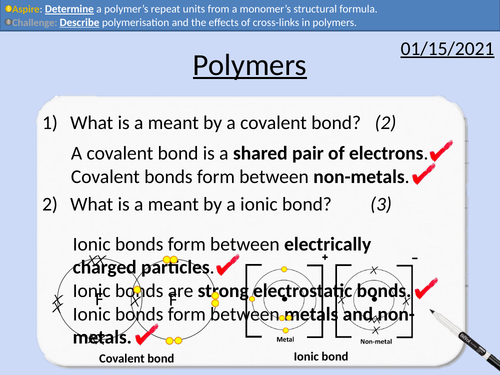
GCSE Chemistry: Polymers
This PowerPoint presentation with worked examples and student questions covers:
• State what is meant by mono- and poly-.
• Describe polymerisation and the effects of cross-links in polymers.
• Determine a polymer’s repeat units from a monomer’s structural formula.
Bundle

OCR A level Chemistry: Aromatic Compounds
OCR A level Chemistry: Aromatic Compounds is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks
Molecular, empirical, skeletal formula for benzene.
The Kekulé model for benzene
Evidence against the Kekule model
The delocalised model for benzene
Nomenclature for benzene rings and aromatic (arene) compounds
Naming benzene containing compounds
Drawing benzene containing compounds
Defining an electrophile
Substitution reactions
Nitration of Benzene
Reaction mechanisms
Halogenation of Benzene
Common Halogen Carriers
Friedel-Crafts Alkylation Reactions
Acyl Chloride
Acylation Reactions of Benzene
Reactivity of Alkenes and Arenes
Naming phenols
Distinguishing between phenols and alcohols
Distinguishing between phenols and alkenes
Distinguishing between phenols and carboxylic acids
Phenol as a weak acid
Electrophilic reactions with phenols
Comparing and explaining the reactivity of phenols and benzene
Naming positions on the aromatic ring
Activating groups and deactivating groups
2-and-4-directing and 3-directing groups
ortho-and-para directing and meta directing groups
Two-step synthesis routes for benzene using directing groups.
Nitration of benzene
Halogenation of benzene
Friedel-Crafts Alkylation of benzene

A Level Chemistry: Carbonyl Compounds
OCR A level Chemistry: 26.1 Carbonyl Compounds
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
The carbonyl group
Differentiating between aldehydes and ketones
Naming aldehydes and ketones
Oxidation of aldehydes
Electronegativity and polar bonds
Electrophiles, nucleophiles, and nucleophilic addition reactions
Reducing carbonyl compounds with sodium tetrahydridoborate(III) (NaH4)
Primary and secondary alcohols from carbonyl compounds
Reacting carbonyl compounds with hydrogen cyanide (HCN)
Reaction mechanisms for nucleophilic addition using (NaBH4)
Reaction mechanisms for nucleophilic addition using (HCN)