496Uploads
162k+Views
70k+Downloads
Chemistry

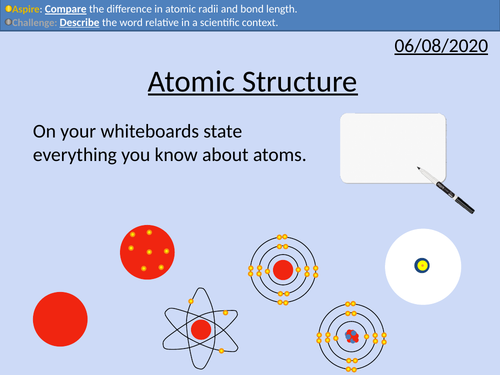
GCSE Chemistry: Atomic Structure
This PowerPoint presentation with worked examples and student questions covers:
• Scientific models as a concept
• Structure of the atom
• Relative mass and charge of subatomic particles
• Bond length of atoms and molecules

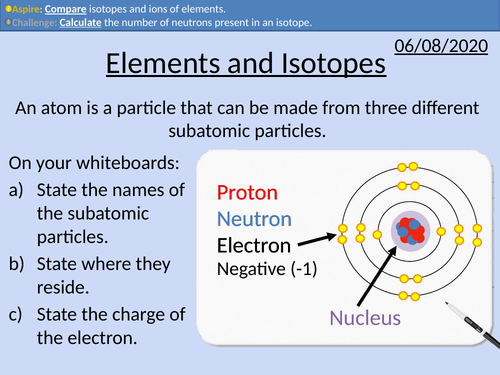
GCSE Chemistry: Isotopes and Ions
This PowerPoint presentation with worked examples and student questions covers:
• Definitions of elements, isotopes, and ions
• State mass number, atomic number, and chemical symbols
• Calculate the number of neutrons
Bundle

GCSE OCR Chemistry: P1.1 The Particle Model
All resources for P1.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Introducing Particles
Chemical and Physical Changes
Limitations of the Particle Model
Bundle

GCSE OCR Chemistry: P1.2 Atomic Structure
All resources for P1.2 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

GCSE Chemistry: Development of the Atomic Model
This PowerPoint presentation with worked examples and student questions covers:
• Dalton, Thomson, Rutherford and Bohr’s models
• Comparing different scientific models of the atom
Bundle

GCSE OCR Chemistry C1 Particles
All resources for P1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Introducing Particles
Chemical and Physical Changes
Limitations of the Particle Model
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

GCSE Chemistry: Relative Formula Mass
This PowerPoint presentation with worked examples and student questions covers:
• Relative atomic mass
• Understanding chemical formulas
• Relative formula mass

OCR Applied Science: 1.3 Ionic and Covalent Bonding
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.3 of Science Fundementals of the OCR Applied Science Spec.
Elements react together to form compounds by i.e.
ionic bonding
covalent bonding

GCSE Chemistry: Empirical Formula
This PowerPoint presentation with worked examples and student questions covers:
• Calculate empirical formula and by finding the simplest whole-number ratio
• Calculate relative formula mass from balanced equations.

OCR Applied Science: 1.2 The Periodic Table
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.2 of Science Fundementals of the OCR Applied Science Spec.
Elements are based on atomic structure and can be classified by the Periodic Table i.e.:
organisation of elements within the table
groups
periods
atomic number
atomic mass atomic radius

GCSE Chemistry: Filtration and Crystallisation
This PowerPoint presentation with worked examples and student questions covers:
Definitions for solution, solute, solvent, insoluble, soluble.
The technique of filtration
The technique of crystallisation

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Paper Chromatography & Rf Values
This PowerPoint presentation with worked examples and student questions covers:
• Definition of technique for paper chromatography
• Experimental procedure
• Definitions of stationary and mobile phase
• Application of Rf equation with examples and answers

OCR Applied Science: 6.2 Physico-chemical Properties of Materials
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Structure of metals, giant covalent, and simple molecular structures.
Properties of metals, giant covalent, and simple molecular structures.
Forces and bonds of metals, giant covalent, and simple molecular structures.
Phase diagrams – interpreting and calculating changes.
Sublimation and phase diagrams.

GCSE Chemistry: Simple Distillation
This PowerPoint presentation with worked examples and student questions covers:
• Changes of state
• The technique of simple distillation
• Concentration of solute increasing in distillation
• Jobs related to chemistry
• Key word test Insoluble, Soluble, Solvent, Solute, Solution, Distillation, Filtration, and Crystallisation

GCSE Chemistry: Purification and Checking Purity
This PowerPoint presentation with worked examples and student questions covers:
• Choosing the correct separation technique
• Comparisons of mobile and stationary phases for chromatography
• Rf Values
• Analysing chromatographs in gas chromatography

GCSE Chemistry: Thin Layer and Gas Chromatography
This PowerPoint presentation with worked examples and student questions covers:
• Experimental Procedure for Thin Layer Chromatography
• Analysing and calculating Rf Values
• Pros and cons of paper and TL chromatography
• Experimental procedure for Gas Chromatography
• Persuasive writing and embedding literacy in science

OCR Applied Science: 4.2 Polymers and Carbon Compounds
This PowerPoint presentation with worked examples and student activities covers:
Topic 4.2 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Determining the empirical formula for compounds
Draw monomers and repeat units using structural and skeletal formula of the following polymers:
Polyethene
Polypropene
Polylactate
Polystyrene
Polyvinyl chloride (PVC)

GCSE Chemistry: Electronic Structures
This PowerPoint presentation with worked examples and student questions covers:
• Electrons reside in energy levels (shells) around the nucleus
• The electronic configuration of elements up to 20 is 2,8,8,2
• Groups and periods of the periodic table
• Drawing electron configurations

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).