475Uploads
143k+Views
63k+Downloads
Chemistry

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces
Bundle

OCR AS level Chemistry: Alkanes
OCR AS level Chemistry: Alkanes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Bundle

OCR A level Chemistry: Carbonyl and Carboxylic Acids
OCR A level Chemistry: Aromatic Compounds is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks
26.1 Carbonyl Compounds
26.2 Identifying Aldehydes and Ketones
26.3 Carboxylic Acids
26.4 Carboxylic Acid Derivatives
The carbonyl group
Differentiating between aldehydes and ketones
Naming aldehydes and ketones
Oxidation of aldehydes
Electronegativity and polar bonds
Electrophiles, nucleophiles, and nucleophilic addition reactions
Reducing carbonyl compounds with sodium tetrahydridoborate(III) (NaH4)
Primary and secondary alcohols from carbonyl compounds
Reacting carbonyl compounds with hydrogen cyanide (HCN)
Reaction mechanisms for nucleophilic addition using (NaBH4)
Reaction mechanisms for nucleophilic addition using (HCN)
Testing for Carbonyl Groups
Brady’s reagent - 2,4-dinitrophenylhydrazine - 2,4-DNP
Distinguishing between Aldehydes and Ketones
Tollen’s reagent - silver nitrate in aqueous ammonia
The Carboxyl Group and polarity of bonds.
Naming carboxylic acids
Carboxylic acids as weak acids
Reactions of carboxylic acids with:
Metals
Metal oxides
Alkali
Carbonates
Changing solubility of carboxylic acids in water due to carbon chain length.
Naming acyl chlorides
Naming acid anhydrides
Naming esters
Esterification
Acid hydrolysis of esters
Alkali hydrolysis of esters
Producing acyl chlorides from carboxylic acids
Producing carboxylic acids from acyl chlorides
Producing esters from acyl chlorides and phenols
Primary, secondary, and tertiary molecules
Producing primary amides from acyl chlorides
Producing secondary amides with acyl chlorides
Producing esters and carboxylic acids wirh acid anhydride

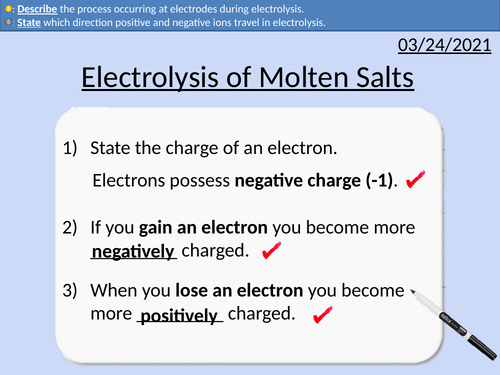
GCSE Chemistry: Electrolysis of molten salts
This PowerPoint presentation with worked examples and student questions covers:
• Naming electrolysis experimental set up
• PANIC convention for electrodes
• Electron transfers at electrodes
• Half-equations for anode and cathode

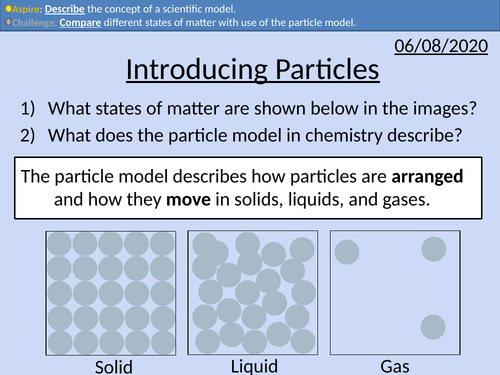
GCSE Chemistry: Introducing Particles
This PowerPoint presentation with worked examples and student questions covers:
• Solids, liquids, and gases
• Scientific models as a concept

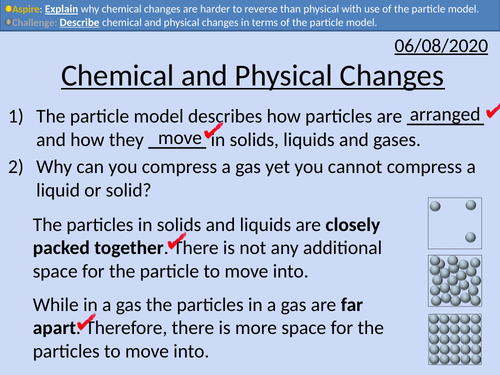
GCSE Chemistry: Chemical and Physical Changes
This PowerPoint presentation with worked examples and student questions covers:
• Differences between physical and chemical changes
• Explain why physical changes are generally easier to reverse

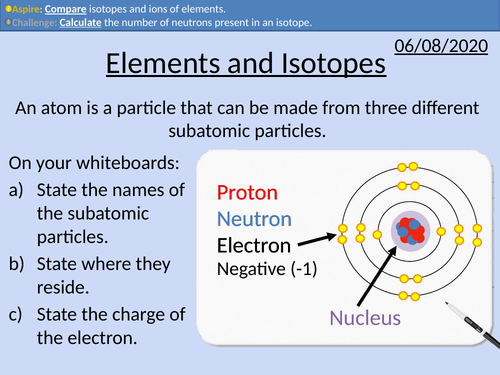
GCSE Chemistry: Isotopes and Ions
This PowerPoint presentation with worked examples and student questions covers:
• Definitions of elements, isotopes, and ions
• State mass number, atomic number, and chemical symbols
• Calculate the number of neutrons
Bundle

GCSE OCR Chemistry: P1.2 Atomic Structure
All resources for P1.2 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Atomic Structure
Isotopes and Ions
Developing the Atomic Model
Bundle

GCSE OCR Chemistry C1 Particles
All resources for P1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Introducing Particles
Chemical and Physical Changes
Limitations of the Particle Model
Atomic Structure
Isotopes and Ions
Developing the Atomic Model

GCSE Chemistry: Relative Formula Mass
This PowerPoint presentation with worked examples and student questions covers:
• Relative atomic mass
• Understanding chemical formulas
• Relative formula mass

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Simple Molecules
This PowerPoint presentation with worked examples and student questions covers:
• Dot and cross diagrams of simple molecules
• Simple molecules form covalent bonds
• The group number on the periodic table informs us how many electrons are in the outer shell.
• Groups on the periodic table

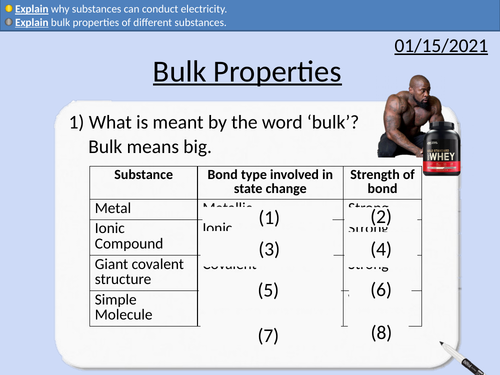
GCSE Chemistry: Bulk Properties
This PowerPoint presentation with worked examples and student questions covers:
• Jobs in Material Science
• Bulk properties of metals - malleable and conductors of electricity
• Bulk properties of ionic and covalent structures - brittle
• Explain why substances conducting electricity depends upon the state of matter

OCR Applied Science: 21.1 Regulatory Bodies
This PowerPoint presentation with worked examples and student activities covers: Topic 1.1 and 1.2 of Module 21: Product Testing Techniques.
Understand the influence of regulatory bodies on development of consumer products.
1.1 The relevant governing bodies that oversee product safety for
manufacturers and consumers of products.
1.2 How governing bodies influence how quality control is applied.

GCSE Chemistry: Carbon
This PowerPoint presentation with worked examples and student questions covers:
• State processes of the carbon cycle.
• Define the word allotrope.
• Explain why allotropes have different properties.
• Graphite, graphene, and fullerenes

GCSE Chemistry: Covalent Structures
This PowerPoint presentation with worked examples and student questions covers:
• Definition of giant covalent structures
• An empirical formula shows the simplest whole-number ratio of the atoms of each compound.
• Melting and boiling point of simple molecules
• Compare physical properties of simple molecules and giant covalent lattices.
Bundle

GCSE OCR Chemistry C2.1 Purity and Separating Mixtures
All resources for P2.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Relative Formula Mass
Empirical Formula
Pure and Impure Substances
Filtration and Crystallisation
Simple Distillation
Paper Chromatography
Gas and Think Layer Chromatography
Purification and Checking Purity

GCSE Chemistry: Group 7 - Halogens
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Halogens
• Trends and anomalies in Group 7 (Density, Melting Point)
• Reactivity of Group 7 Halogens
• Electron configuration of Group 7 Halogens
• Forming salts with alkali metals and halogens

OCR AS Chemistry: Organic Chemistry
OCR AS Chemistry: 11.1 Organic Chemistry
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definition of hydrocarbons
What organic chemistry is
Saturated and unsaturated hydrocarbons
Definition of functional groups
Definition of homologous group
Bundle

OCR AS level Chemistry: Basic Concepts of Organic Chemistry
OCR AS level Chemistry: Basic Concepts of Organic Chemistry apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks