496Uploads
163k+Views
70k+Downloads
All resources
Bundle

OCR AS level Chemistry: Alkenes
OCR AS level Chemistry: Alkenes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.
E/Z isomerism
Conditions for trans- and cis- isomerism
Cahn-Ingold-Prelog rules and priority ordering
Alkene addition reactions:
Hydrogen with a nickel catalyst
Halogens
Hydrogen halide
Steam with an acid catalyst
Test for unsaturated alkenes.
Bond enthalpy for sigma and pi bonds.
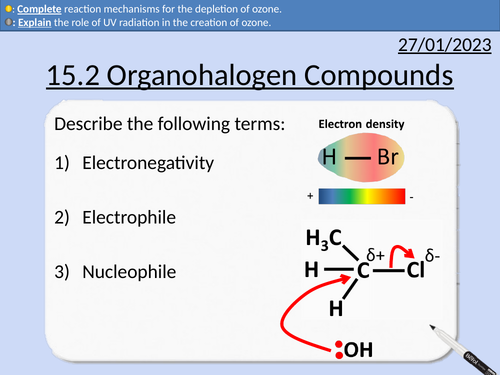
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule
Monomers and repeat units
Addition Polymerisation for:
Polyethene
Polypropene
Polylactate
Polystyrene
Polyvinyl Chloride (PVC)
Environmental Concerns from polymers including:
Combustion of polymers
recycling PVC
biogradeable bioplastics
photodegradable polymers
feedstock recycling

OCR AS Chemistry: Electrophilic Addition in Alkenes
OCR AS Chemistry: 13.4 Electrophilic Addition in Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule

OCR AS Chemistry: Properties of Alcohols
OCR AS Chemistry: 14,1 Properties of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces

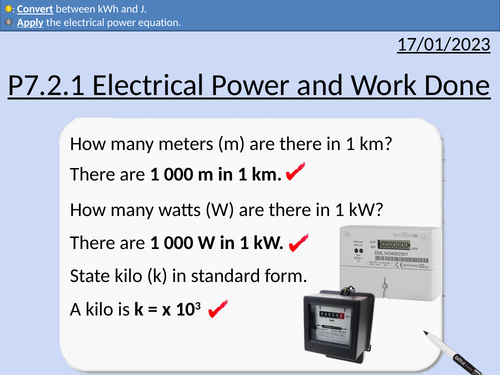
GCSE Physics: Electrical Power and Work Done
This presentation covers OCR Gateway Physics 9-1 P7.2.1 Electrical Power and Work Done. All presentations come with student activities and worked solutions.
Definition of power
Converting between W and kW
Converting between seconds, minutes, and hours
Calculating work done in kWh and J
Converting between kWh and J
Bundle

GCSE OCR Physics: P7.2 Power and Efficiency
All resources for P7.2 P7.2 Power and Efficiency GCSE OCR Physics Gateway 9-1. Triple and combined (Higher and Foundation) is covered in this material.
All powerpoints include student activities and worked examples.
Electrical Work Done
Paying for Electricity
Electrical Energy Transfers
Electrical Heating
Thermal Conductivity
Efficiency and Sankey Diagrams

GCSE Physics: Electrical Heating
This presentation covers OCR Gateway Physics 9-1 P7.2.3 Electrical Heating Transfers. All presentations come with student activities and worked solutions.
Walls and Insulation
Thermal energy dissipation
Reducing thermal energy dissipation with lubrication and insulation
Heating substances and state changes
Work done = Power x Time
Change in thermal energy = Mass x Specific Heat Capacity x Change in temperature
Thermal energy for state change = Mass x Specific latent heat

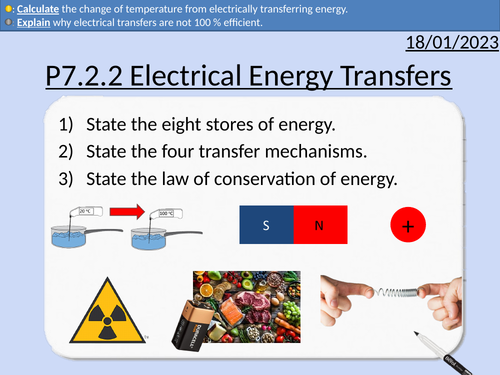
GCSE Physics: Electrical Energy Transfers
This presentation covers OCR Gateway Physics 9-1 P7.2.2 Electrical Energy Transfers. All presentations come with student activities and worked solutions.
Energy stores
Energy transfers
Current heats wires
Wasted energy in motors and heating elements
Specific heat capacity and electrical energy
Thermal energy = Mass x Specific Heat Capacity x Change in Temperature
Energy = Charge x Potential Difference
Bundle

OCR AS level Chemistry: Haloalkanes
OCR AS level Chemistry: Haloalkanes is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Naming Haloalkanes
Classifying Haloalkanes (primary, secondary, tertiary)
Electronegativity
Reaction mechanism for hydrolysis
Rates of reactions for hydrolysis
Reaction conditions for hydrolysis
Definitions for CFC (Chlorofluorocarbons) and HCFC (Hydachlorofluorocarbons)
Creation of ozone
Depletion of ozone with CFCs
Reaction steps including initiations and propagation

GCSE Physics: Efficiency
This presentation covers OCR Gateway Physics 9-1 P7.2.5 Efficiency.
All presentations come with student activities and worked solutions.
Efficiency Ratings
Improving efficiency with insulation and lubrication
Maximum efficiency
Efficiency equation
Sankey diagrams

OCR AS Chemistry: The Chemistry of Haloalkanes
OCR AS Chemistry: The Chemistry of Haloalkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming Haloalkanes
Classifying Haloalkanes (primary, secondary, tertiary)
Electronegativity
Reaction mechanism for hydrolysis
Rates of reactions for hydrolysis
Reaction conditions for hydrolysis

OCR AS Chemistry: Organohalogen Compounds
OCR AS Chemistry: 15.2 Organohalogen Compounds and the Environment
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definitions for CFC (Chlorofluorocarbons) and HCFC (Hydachlorofluorocarbons)
Creation of ozone
Depletion of ozone with CFCs
Reaction steps including initiations and propagation

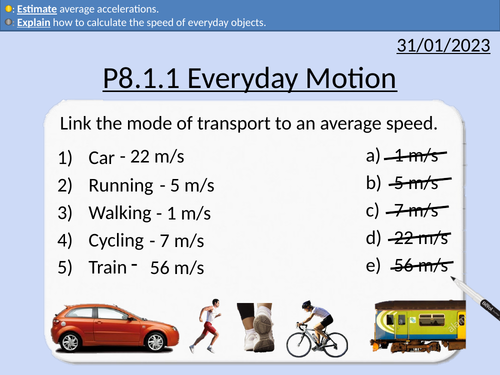
GCSE Physics: Everyday Motion
This presentation covers OCR Gateway Physics 9-1 P8.1.1 Everyday Motion. All presentations come with student activities and worked solutions.
Average speeds of walking, running, cycling, cars, trains, wind, sound, and light.
The speed equation
The acceleration equation
Explaining average speed camera
Explaining instantaneous speed camera
Estimating everyday accelerations
Calculating speed from rotation speed and circumference of wheels
Converting from miles per hour to meters per second

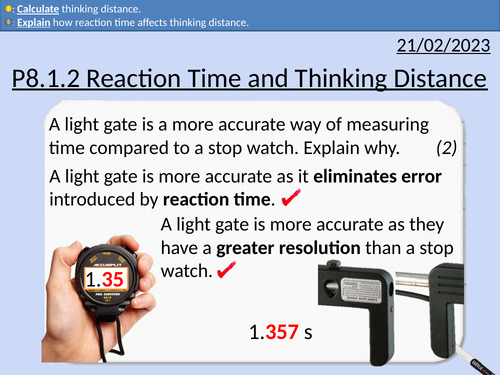
GCSE Physics: Reaction Time and Thinking Distance
This presentation covers OCR Gateway Physics 9-1 P8.1.2 Reaction Time and Thinking Distance. All presentations come with student activities and worked solutions.
Reaction time definition
Factors that increase reaction time
Simple reaction time experiment
Thinking distance
Rearranging equations
Speed equation
(Final velocity)2 – (Initial velocity)2 = 2 x Acceleration x Distance
v2 – u2 = 2 a s
Bundle

GCSE OCR Physics: P8.1 Physics on the move
All resources for P8.1 Physics on the move GCSE OCR Physics Gateway 9-1. Triple and combined (Higher and Foundation) is covered in this material.
Average speeds of walking, running, cycling, cars, trains, wind, sound, and light.
The speed equation
The acceleration equation
Explaining average speed camera
Explaining instantaneous speed camera
Estimating everyday accelerations
Calculating speed from rotation speed and circumference of wheels
Converting from miles per hour to meters per second
Reaction time definition
Factors that increase reaction time
Simple reaction time experiment
Thinking distance
Rearranging equations
Speed equation
(Final velocity)2 – (Initial velocity)2 = 2 x Acceleration x Distance
v2 – u2 = 2 a s
Factors affecting braking distance
Total stopping distances
Calculating area of a velocity-time graph for displacement (distance traveled).
Rearranging equations
MOT testing
Large accelerations produce large forces.
Values of g that cause severe injury or death
Road Safety
Newton’s First Law and seat belts
Crumple zones
Force = Mass x Acceleration
Acceleration = Change in velocity /Time taken
Estimating speed, accelerations and forces involved in large accelerations for everyday road transport.

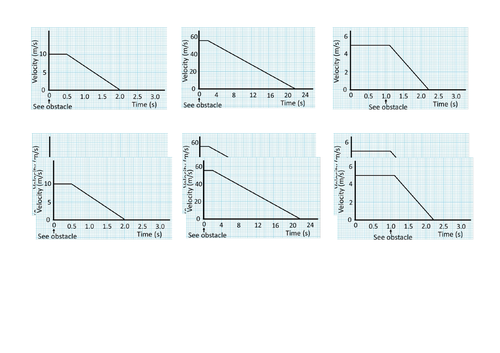
GCSE Physics: Braking and Stopping Distances
This presentation covers OCR Gateway Physics 9-1 P8.1.3 Braking and Stopping Distances. All presentations come with student activities and worked solutions.
Factors affecting braking distance
Total stopping distances
Calculating area of a velocity-time graph for displacement (distance traveled).
Rearranging equations
MOT testing
(Final velocity)2 – (Initial velocity)2 = 2 x Acceleration x Distance
v2 – u2 = 2 a s

GCSE Physics: Forces in Collisions
This presentation covers OCR Gateway Physics 9-1 P8.1.4 Forces in Collisions. All presentations come with student activities and worked solutions.
Large accelerations produce large forces.
Values of g that cause severe injury or death
Road Safety
Newton’s First Law and seat belts
Crumple zones
Force = Mass x Acceleration
Acceleration = Change in velocity /Time taken
Estimating speed, accelerations and forces involved in large accelerations for everyday road transport.

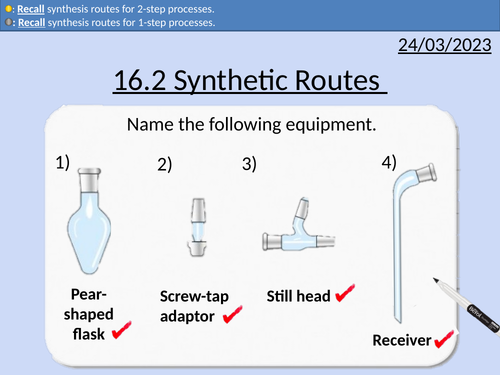
OCR AS Chemistry: Synthetic Routes
OCR AS Chemistry: 16.2 Synthetic Routes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Functional Groups - Alkane, Alkene, Haloalkane, Alcohols, Carboxylic Acid, Ketone, Aldehyde, Ester, Amine, Nitrile.
One-step synthetic routes with reagents and conditions
Two-step synthetic routes with reagents and conditions
Bundle

OCR AS level Chemistry: Organic Synthesis
OCR AS level Chemistry: Organic Synthesis is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Heating under reflux
Distillation
Re-distillation
Purifying Organic Products
Removing impure acids from organic compounds
Drying agents
Functional Groups - Alkane, Alkene, Haloalkane, Alcohols, Carboxylic Acid, Ketone, Aldehyde, Ester, Amine, Nitrile.
One-step synthetic routes with reagents and conditions
Two-step synthetic routes with reagents and conditions
Bundle

OCR AS level Chemistry: Alcohols
OCR AS level Chemistry: Alcohols is apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks
Naming alcohols
Classifying alcohols (primary, secondary, tertiary)
Electronegativity
Polar and non-polar molecules
Explaining physical properties of alcohols compared to alkanes
Volatility
Solubility
Melting points
Chain length and London forces
Combustion of alcohols
Reflux condition for reactions
Primary alcohol to aldehydes
Primary alcohols to carboxylic acids
Secondary alcohols to ketones
Dehydration of alcohols
Substitution reactions for alcohols
Bundle

GCSE OCR Physics: P8.2 Powering Earth
All resources for P8.2 Powering Earth GCSE OCR Physics Gateway 9-1. Triple and combined (Higher and Foundation) is covered in this material.
Types of different energy sources
Renewable and non-renewable definitions
Different uses of energy sources - transport, heating, and generating electricity
Advantages and disadvantages of different energy sources
Fossil fuels – oil, coal, and natural gas.
Nuclear fuel – Uranium
Biofuels – wood, biodiesel, and biogas.
The sun - solar (PV) panels and solar heating panels
Tides
Waves
Hydroelectricity
Wind
Geothermal
How use of energy resources have changed over time. (Biofuels, Fossil Fuels, Nuclear, Renewable).
How energy use has increased (increase population and development of technology)
Explain patterns and trends in the use of energy resources.
Fossil fuels are finite and will run out at current consumption levels.
Structure of the National Grid
Step-up and Step-down transformers
How transformers increase the efficiency of the National Grid
Number of turns and potential difference
Current and potential difference in primary and secondary coils
Domestic Electrical Supply being 230 V, AC at 50 Hz.
Direct potential difference and alternating potential difference.
Reasons for insulation on wires.
Potential Difference between different conductors.
Function of the earth conductor.
Double insulation and no earth wire.
Reasons the live wire is dangerous.
Reasons why live to earth is dangerous.