497Uploads
167k+Views
71k+Downloads
Chemistry

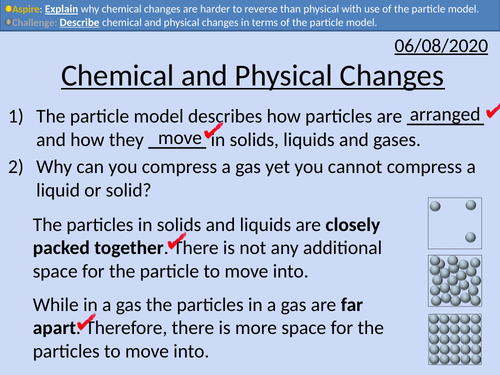
GCSE Chemistry: Chemical and Physical Changes
This PowerPoint presentation with worked examples and student questions covers:
• Differences between physical and chemical changes
• Explain why physical changes are generally easier to reverse

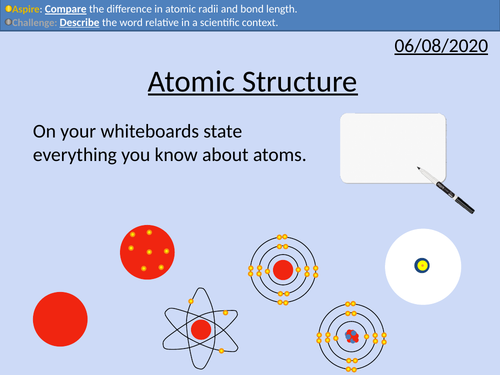
GCSE Chemistry: Atomic Structure
This PowerPoint presentation with worked examples and student questions covers:
• Scientific models as a concept
• Structure of the atom
• Relative mass and charge of subatomic particles
• Bond length of atoms and molecules

GCSE Chemistry: Pure and Impure Substances
This PowerPoint presentation with worked examples and student questions covers:
Definitions of pure and impure substances
Definition of an alloy
Identification of purity with melting points
Plotting graphs and data analysis

GCSE Chemistry: Simple Distillation
This PowerPoint presentation with worked examples and student questions covers:
• Changes of state
• The technique of simple distillation
• Concentration of solute increasing in distillation
• Jobs related to chemistry
• Key word test Insoluble, Soluble, Solvent, Solute, Solution, Distillation, Filtration, and Crystallisation

GCSE Chemistry: Purification and Checking Purity
This PowerPoint presentation with worked examples and student questions covers:
• Choosing the correct separation technique
• Comparisons of mobile and stationary phases for chromatography
• Rf Values
• Analysing chromatographs in gas chromatography

GCSE Chemistry: Thin Layer and Gas Chromatography
This PowerPoint presentation with worked examples and student questions covers:
• Experimental Procedure for Thin Layer Chromatography
• Analysing and calculating Rf Values
• Pros and cons of paper and TL chromatography
• Experimental procedure for Gas Chromatography
• Persuasive writing and embedding literacy in science

GCSE Chemistry: Nanoparticles
This PowerPoint presentation with worked examples and student questions covers:
• Relative size of nanoparticles
• Convert nanometres using standard form
• Uses and dangers of nanoparticles

OCR AS Chemistry: Chemical Reactions of Alkanes
OCR AS Chemistry: 12.2 Chemical Reactions of Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Combustion reactions
Incomplete combustion reactions
Balancing equations
Using algebraic equations
Radical substitution reactions
Reaction mechanism for haloalkanes - Initiation, Propagation, and Termination
Monosubstituted (positional isomers) isomers

GCSE Chemistry: Relative Formula Mass
This PowerPoint presentation with worked examples and student questions covers:
• Relative atomic mass
• Understanding chemical formulas
• Relative formula mass

GCSE Chemistry: Empirical Formula
This PowerPoint presentation with worked examples and student questions covers:
• Calculate empirical formula and by finding the simplest whole-number ratio
• Calculate relative formula mass from balanced equations.

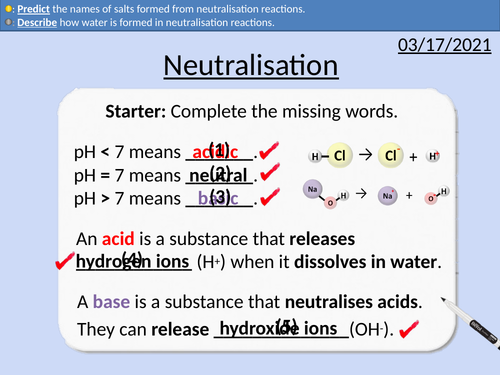
GCSE Chemistry: Neutralisation Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Word equations for neutralisation reactions
• Describing how ions form salts
• Describing how water is formed
• Predicting the names of salts formed

GCSE Chemistry: Development of the Atomic Model
This PowerPoint presentation with worked examples and student questions covers:
• Dalton, Thomson, Rutherford and Bohr’s models
• Comparing different scientific models of the atom

GCSE Chemistry: Metals and Non-metals
This PowerPoint presentation with worked examples and student questions covers:
• Using the periodic table to identify metals and non-metals
• Different properties of metal and non-metals (Appearance, melting and boiling point, state of matter at room temperature, ductility, and malleability).
• Exceptions of physical properties (mercury being liquid and carbon conducting electricity).

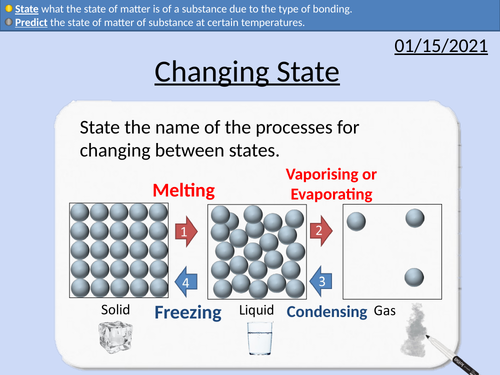
GCSE Chemistry: Changing State
This PowerPoint presentation with worked examples and student questions covers:
• Define melting and boiling point of a pure substance.
• Predict the state of matter of substance at certain temperatures.
• State what the state of matter is of a substance due to the type of bonding.
• Metals, covalent structures, ionic structures and simple molecules.

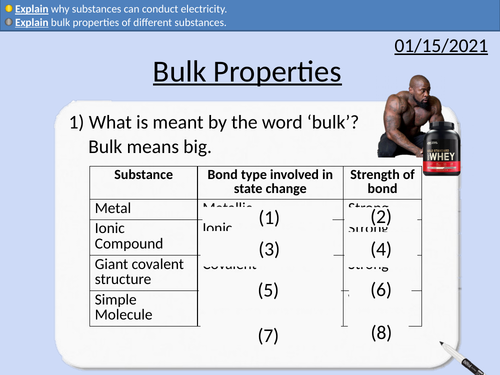
GCSE Chemistry: Bulk Properties
This PowerPoint presentation with worked examples and student questions covers:
• Jobs in Material Science
• Bulk properties of metals - malleable and conductors of electricity
• Bulk properties of ionic and covalent structures - brittle
• Explain why substances conducting electricity depends upon the state of matter

GCSE Chemistry: Formulae of Elements and Molecules
This PowerPoint presentation with worked examples and student questions covers:
• State the number of elements in a chemical formula.
• Determine the chemical formula from display formula.
• Dot and cross diagrams for bonded atoms

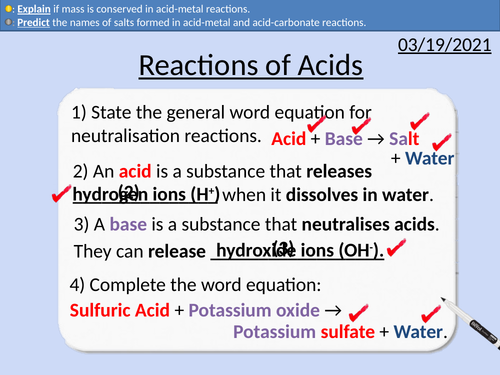
GCSE Chemistry: Reactions of Acids
This PowerPoint presentation with worked examples and student questions covers:
• Identifying metals on the periodic table
• Predicting the salt formed in acid metal reactions.
• Predicting the salt formed in acid carbonate reactions.
• Conservation of mass and state symbols

GCSE Chemistry: Transition Metals
This PowerPoint presentation with worked examples and student questions covers:
• Properties of transition metals gases
• Comparing transition metals with alkali metals
• Everyday applications of transition metals
• Transition metals as catalysts

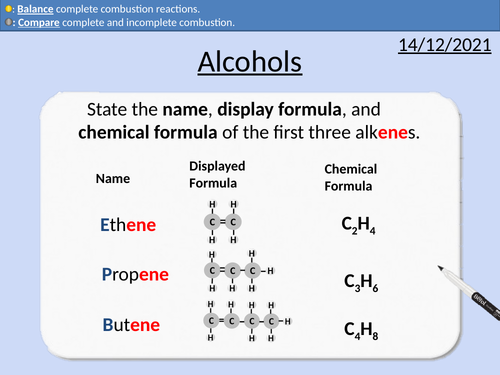
GCSE Chemistry: Alcohols
This PowerPoint presentation with worked examples and student questions covers:
• Functional groups of alcohols, alkanes, and alkenes.
• Comparing incomplete and complete combustion of alcohols
• Mnemonic device for naming alcohols
• General formula for alcohols
• Drawing the structural formula for alcohols

OCR AS Chemistry: Electrophilic Addition in Alkenes
OCR AS Chemistry: 13.4 Electrophilic Addition in Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Electrophile molecules
Electronegativity
Reaction mechanisms for addition reaction of alkenes and hydrogen halides
Carbocations and stability
Markownikoff’s Rule