497Uploads
167k+Views
71k+Downloads
Chemistry
Bundle

OCR A level Chemistry: Amines, Amino Acids, and Polymers
OCR A level Chemistry: Aromatic Compounds is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks.
27.1 Amines
27.2 Amino acids, Amides and Chirality
27.3 Condensation Polymers
Aliphatic and aromatic hydrocarbons
Amines being derived from ammonia (NH3)
Classifying amines as primary, secondary, and tertiary
Naming amines
Naming ammonium salts
Amines neutralisation reactions with acids
Preparation of aliphatic amines
Preparation of aromatic amines
Locants: alpha, beta, and gamma
Functional groups of amino acids
General formula for amino acids
Reactions of amino acids (alkali and acid)
Esterification of amino acids
Amide functional groups
Naming amide molecules
Drawing optical isomers
Explanation of superimposable and non-superimposable images
Identifying chiral centers
Recap of addition polymerisation
Identifying monomers and repeat units from condensation polymers
Polyesters and ester links
Polyamides and amide links
Polyesters and polyamides formed from one monomer
Polyesters and polyamide formed from two monomers
Alkali hydrolysis of polyamides and polyesters
Acid hydrolysis of polyamides and polyesters
Bundle

OCR A level Chemistry: Chromatography and Spectroscopy
OCR A level Chemistry: Chromatography and Spectroscopy is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks.
29.1 Chromatography and Functional Group Analysis
29.2 Nuclear Magnetic Resonance (NMR) Spectroscopy
29.3 Carbon-13 NMR Spectroscopyy
29.4 Proton NMR Spectroscopy
29.5 Interpreting Proton NMR Spectra
29.6 Combined Techniques
Thin layer chromatography (TLC)
Rf values
Gas chromatography (GC)
Gas chromatograms
Retention time and peak integrations
Calibration curves from retention time and relative peak area
Differentiation of functional groups: alkene, primary and secondary alcohols, aldehydes, cabonyl compounds, carboxylic acids, and haloalkes.
Nuclear Spin
Resonance
Tetramethylsilane (TMS)
Chemical Shift ẟ
Identifying different carbon environments
The types of carbon environment
The amount of chemical shift ẟ / ppm
Identifying the number of different proton environments
Identifying the types of proton environment and chemical shifts
Integration traces (area of peaks) and relative number of protons
The spin-spin splitting pattern (n + 1)
Predicting proton NMR spectra for molecules
Identifying the number of different proton environments
Identifying the types of proton environment and chemical shifts
Integration traces (area of peaks) and relative number of protons
Percentage yield to determine empirical formula
Mass spectra
Infrared spectra
Carbon-13 NMR spectra
Proton NMR spectra

OCR AS Chemistry: Introduction to Reaction Mechanisms
OCR AS Chemistry: 11.5 Introduction to Reaction Mechanisms
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Covalent bonds
Homolytic fission and heterolytic reactions
Curly arrows in reaction mechanisms
Identifying addition, substitution, and elimination reactions.

OCR AS Chemistry: Alkanes
OCR AS Chemistry: 12.1 Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Sigma bonds (σ-bonds).
Tetrahedral shape and bond angles
Fractional distillation
Chain length and boiling point
Branching and boiling point
London Forces

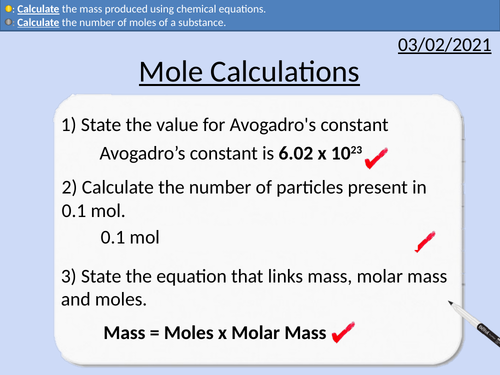
GCSE Chemistry: Mole Calculations
This PowerPoint presentation with worked examples and student questions covers:
• Rearranging Equations
• Stoichiometry as relative abundances
• Relative Atomic Mass, Relative Formula Mass and Molar Mass
• Calculating the number of moles present
• Conservation of mass
Bundle

OCR A level Chemistry: Organic Synthesis
OCR A level Chemistry: Organic Synthesis is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks.
28.1 Carbon-Carbon Bond Formation
28.2 Further Practical Techniques
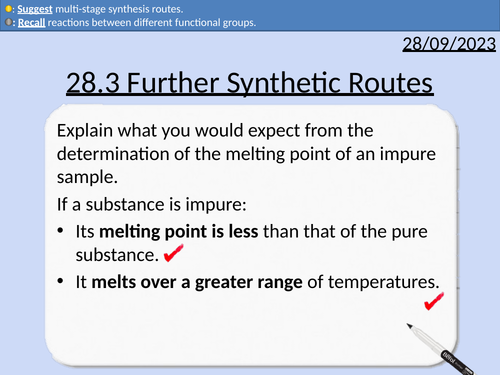
28.3 Further Synthetic Routes
Forming nitriles from haloalkanes
Forming nitriles from aldehydes and ketones
Forming amines from nitriles (reduction)
Forming carboxylic acids from nitriles (hydrolysis)
Friedel-Crafts alkylation of benzene
Acylation of benzene with acyl chloride
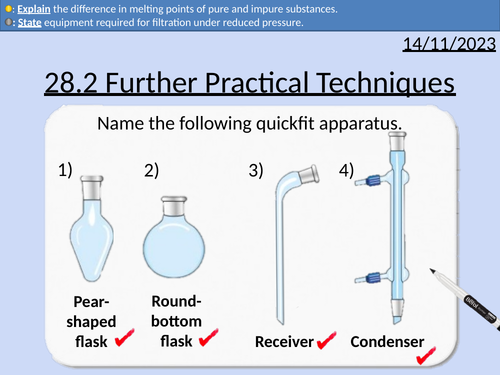
Filtration under reduced pressure
Purification through Recrystallisation
Preparation of Melting Point Sample
Melting point determination with an electric heater
Melting point determination with a Thiele tube
Functional groups
Reactions of benzenes
Reactions of phenols
Common reactions between different functional groups
Reaction conditions and reagents

A level Chemistry: Further Practical Techniques
OCR A level Chemistry: 28.2 Further Practical Techniques
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Filtration under reduced pressure
Purification through Recrystallisation
Preparation of Melting Point Sample
Melting point determination with an electric heater
Melting point determination with a Thiele tube

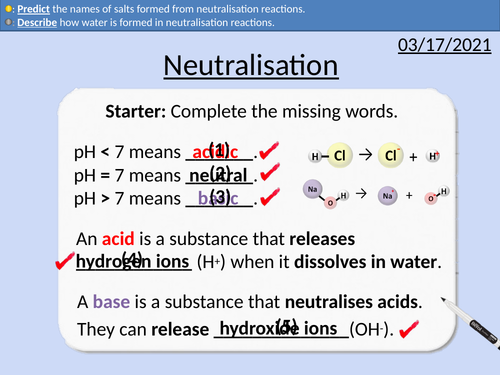
GCSE Chemistry: Neutralisation Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Word equations for neutralisation reactions
• Describing how ions form salts
• Describing how water is formed
• Predicting the names of salts formed

OCR AS Chemistry: Structural Isomerism
OCR AS Chemistry: 11.4 Structural Isomerism
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definition for Structural Isomers
Moving functional group to form isomers
Aldehydes and ketones being structural isomers
Skeletal formula and structural formula

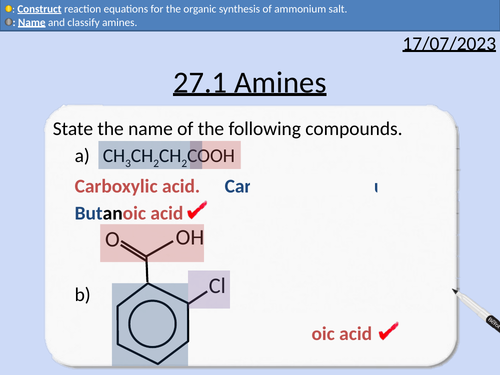
A level Chemistry: Amines
OCR A level Chemistry: 27.1 Amines
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Aliphatic and aromatic hydrocarbons
Amines being derived from ammonia (NH3)
Classifying amines as primary, secondary, and tertiary
Naming amines
Naming ammonium salts
Amines neutralisation reactions with acids
Preparation of aliphatic amines
Preparation of aromatic amines
Bundle

OCR A level Chemistry: Carbonyl and Carboxylic Acids
OCR A level Chemistry: Aromatic Compounds is apart of the Module 6: Organic Chemistry and Analysis.
All presentations come with worked examples, solutions and homeworks
26.1 Carbonyl Compounds
26.2 Identifying Aldehydes and Ketones
26.3 Carboxylic Acids
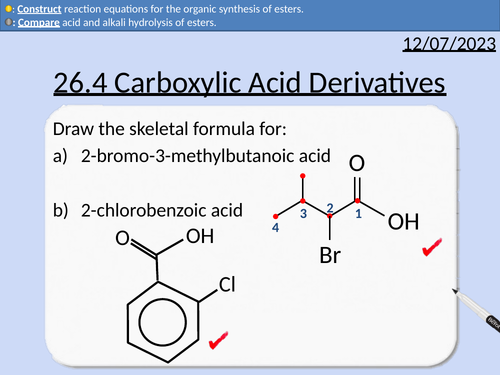
26.4 Carboxylic Acid Derivatives
The carbonyl group
Differentiating between aldehydes and ketones
Naming aldehydes and ketones
Oxidation of aldehydes
Electronegativity and polar bonds
Electrophiles, nucleophiles, and nucleophilic addition reactions
Reducing carbonyl compounds with sodium tetrahydridoborate(III) (NaH4)
Primary and secondary alcohols from carbonyl compounds
Reacting carbonyl compounds with hydrogen cyanide (HCN)
Reaction mechanisms for nucleophilic addition using (NaBH4)
Reaction mechanisms for nucleophilic addition using (HCN)
Testing for Carbonyl Groups
Brady’s reagent - 2,4-dinitrophenylhydrazine - 2,4-DNP
Distinguishing between Aldehydes and Ketones
Tollen’s reagent - silver nitrate in aqueous ammonia
The Carboxyl Group and polarity of bonds.
Naming carboxylic acids
Carboxylic acids as weak acids
Reactions of carboxylic acids with:
Metals
Metal oxides
Alkali
Carbonates
Changing solubility of carboxylic acids in water due to carbon chain length.
Naming acyl chlorides
Naming acid anhydrides
Naming esters
Esterification
Acid hydrolysis of esters
Alkali hydrolysis of esters
Producing acyl chlorides from carboxylic acids
Producing carboxylic acids from acyl chlorides
Producing esters from acyl chlorides and phenols
Primary, secondary, and tertiary molecules
Producing primary amides from acyl chlorides
Producing secondary amides with acyl chlorides
Producing esters and carboxylic acids wirh acid anhydride

A Level Chemistry: Directing Group for Benzene
OCR A level Chemistry: 25.4 Directing Group
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming positions on the aromatic ring
Activating groups and deactivating groups
2-and-4-directing and 3-directing groups
ortho-and-para directing and meta directing groups
Two-step synthesis routes for benzene using directing groups.
Nitration of benzene
Halogenation of benzene
Friedel-Crafts Alkylation of benzene

OCR AS Chemistry: Representing the formulae of Organic Compounds
OCR AS Chemistry: Formulae for Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
molecular formula
empirical formula
general formula
displayed formula
structural formula
skeletal formula

OCR AS Chemistry: Chemical Reactions of Alkanes
OCR AS Chemistry: 12.2 Chemical Reactions of Alkanes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Combustion reactions
Incomplete combustion reactions
Balancing equations
Using algebraic equations
Radical substitution reactions
Reaction mechanism for haloalkanes - Initiation, Propagation, and Termination
Monosubstituted (positional isomers) isomers

A level Chemistry: Further Synthetic Routes
OCR A level Chemistry: 28.3 Further Synthetic Routes
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Functional groups
Reactions of benzenes
Reactions of phenols
Common reactions between different functional groups
Reaction conditions and reagents

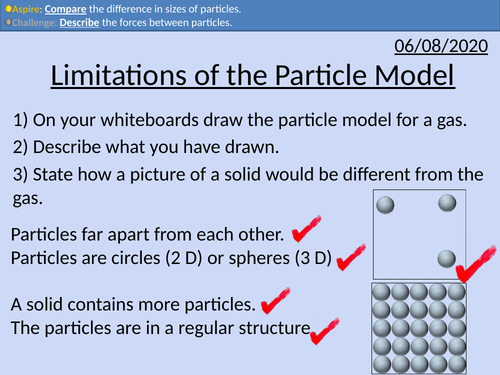
GCSE Chemistry: Limitations of the Particle Model
This PowerPoint presentation with worked examples and student questions covers:
• Describing the limitations of the model: lack of forces between particles, size of particles, and space between the particles.
• Mathematically comparing sizes and distances of particles

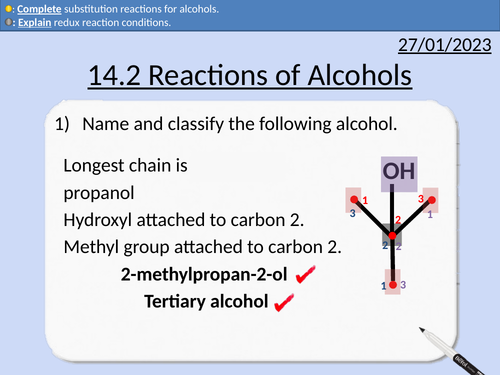
OCR AS Chemistry: Reactions of Alcohols
OCR AS Chemistry: 14.2 Reactions of Alcohols
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Combustion of alcohols
Reflux condition for reactions
Primary alcohol to aldehydes
Primary alcohols to carboxylic acids
Secondary alcohols to ketones
Dehydration of alcohols
Substitution reactions for alcohols

A level Chemistry: Carboxylic Acid Derivatives
OCR A level Chemistry: 26.3 Carboxylic Acids
This PowerPoint is a whole lesson included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Naming acyl chlorides
Naming acid anhydrides
Naming esters
Esterification
Acid hydrolysis of esters
Alkali hydrolysis of esters
Producing acyl chlorides from carboxylic acids
Producing carboxylic acids from acyl chlorides
Producing esters from acyl chlorides and phenols
Primary, secondary, and tertiary molecules
Producing primary amides from acyl chlorides
Producing secondary amides with acyl chlorides
Producing esters and carboxylic acids wirh acid anhydride

GCSE Chemistry: Alkenes
This PowerPoint presentation with worked examples and student questions covers:
• Unsaturated hydrocarbons
• Comparing alkanes and alkenes
• Mnemonic device for naming alkenes
• General formula for alkenes
• Completing addition reactions for alkenes

OCR AS Chemistry: Properties of Alkenes
OCR AS Chemistry: 13.1 Properties of Alkenes
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Comparing pi-bond (π-bond) and sigma bonds (σ-bonds).
Aliphatic alkenes and alicyclic arrangements of molecules
s, p, d orbitals for electrons
Trigonal planar shape of alkanes leading to 120 degree bond angle.