497Uploads
167k+Views
71k+Downloads
Chemistry

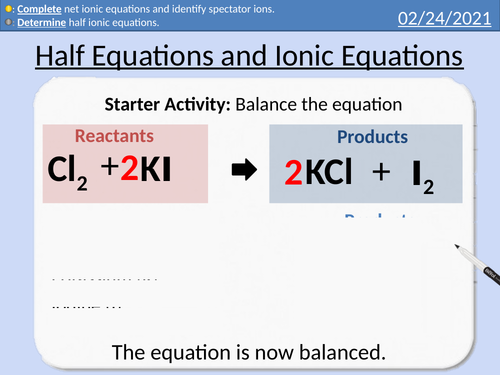
GCSE Chemistry: Half Equations and Ionic Equations
This PowerPoint presentation with worked examples and student questions covers:
• Precipitation in chemical reactions
• Definition of ions
• Ionic Half equations
• Dot and cross diagrams for electron structure
• Introduction to full ionic equations and net ionic equations

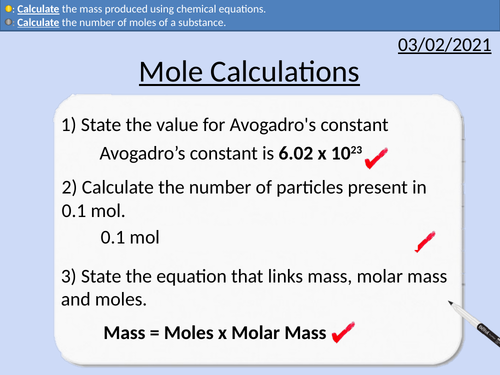
GCSE Chemistry: Mole Calculations
This PowerPoint presentation with worked examples and student questions covers:
• Rearranging Equations
• Stoichiometry as relative abundances
• Relative Atomic Mass, Relative Formula Mass and Molar Mass
• Calculating the number of moles present
• Conservation of mass
Bundle

GCSE OCR Chemistry C2.3 Properties of Materials
Resources for C2.3 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Carbon
Changing State
Bulk Properties
Nanoparticles

GCSE Chemistry: Bond Energies and Energy Changes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of bond energies
• Calculating bond energies per mole
• Calculating change in bond energies in reactions
• Determining if a reaction is exothermic or endothermic from the change in bond energy.
Bundle

GCSE OCR Chemistry C3.1 Introducing Chemical Reactions
Resources for C3.1 GCSE OCR Chemistry Gateway 9-1 Triple and Combined (Higher and Foundation) is covered in this material.
Includes:
Formulae of elements and molecules
Formulae of ionic compounds
Conservation of mass
Chemical Equations
Half equations and ionic equations
The mole
Mole calculations

GCSE Chemistry: Exothermic and Endothermic Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition for exothermic and endothermic
• Examples of exothermic and endothermic reactions
• Practical procedure for NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
• Determining if experimental evidence show a exothermic or endothermic reaction

GCSE Chemistry: The pH scale
This PowerPoint presentation with worked examples and student questions covers:
• pH 0 - 14 scale with household examples
• Definitions for acids, bases and alkali substances
• Universal indicator and pH probes
• Using equalities and inequalities

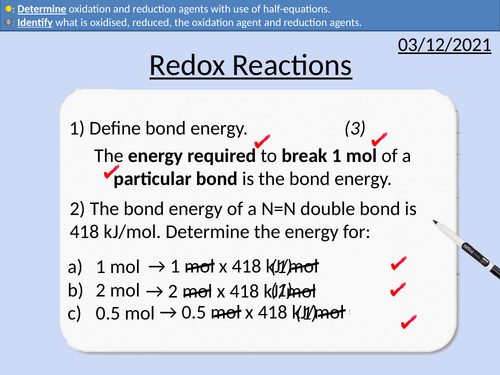
GCSE Chemistry: Redox Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Oxidation and reduction reactions for oxygen
• Identification of oxidation and reduction agents
• Oxidation and reduction reactions for electrons
• Half equations to determine oxidation and reduction

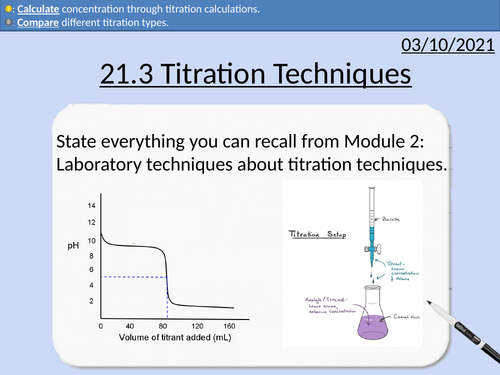
OCR Applied Science: 21.3 Titration Techniques
OCR Applied Science Level 3 - Module 21: Product Testing Techniques.
3.1 Titration techniques on consumer products
• Acid-base titration (e.g. limescale removers, eco-disinfectants)
• Precipitation titration (e.g. contact lens saline solution)
• Redox titration, (e.g. bleach, tooth whitener; vitamin C tablets).
• Complexometric titrations (e.g. Milk of Magnesia)
Including explanation and activities on:
Titration calculations
Moles and molar mass
Rearranging Equations
State symbols
Significant Figures
Comparing Data

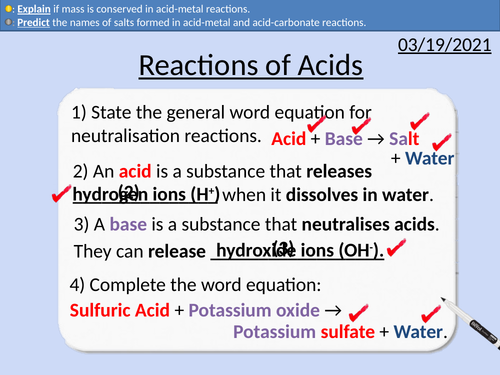
GCSE Chemistry: Reactions of Acids
This PowerPoint presentation with worked examples and student questions covers:
• Identifying metals on the periodic table
• Predicting the salt formed in acid metal reactions.
• Predicting the salt formed in acid carbonate reactions.
• Conservation of mass and state symbols

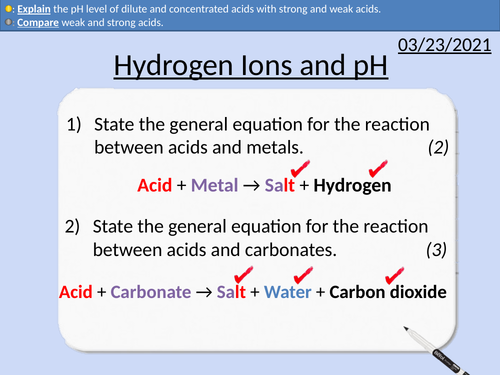
GCSE Chemistry: Hydrogen Ions and pH
This PowerPoint presentation with worked examples and student questions covers:
• Concentration of fruit squash
• Comparing strong and weak acids
• pH and hydrogen ion concentration
• Titration curves

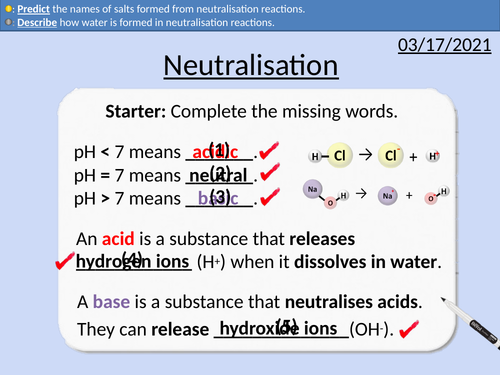
GCSE Chemistry: Neutralisation Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Word equations for neutralisation reactions
• Describing how ions form salts
• Describing how water is formed
• Predicting the names of salts formed

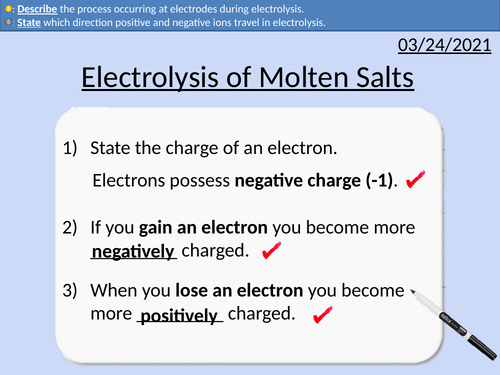
GCSE Chemistry: Electrolysis of molten salts
This PowerPoint presentation with worked examples and student questions covers:
• Naming electrolysis experimental set up
• PANIC convention for electrodes
• Electron transfers at electrodes
• Half-equations for anode and cathode

GCSE Chemistry: Electrolysis of Water
This PowerPoint presentation with worked examples and student questions covers:
• Pure water being made partially of ions (hydrogen and hydroxide).
• PANIC convention for electrodes
• OILRIG convention for redox reactions
• Electron transfers at electrodes
• Half-equations for anode and cathode
• Balancing half-equations

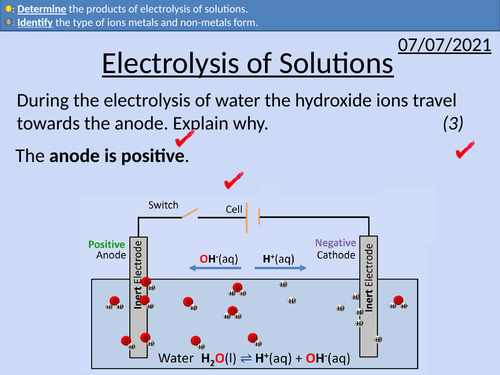
GCSE Chemistry: Electrolysis of Solutions
This PowerPoint presentation with worked examples and student questions covers:
• The position of metals and non-metals on the periodic table
• The ions metals and non-metals form
• The ion composition of solutions
• Electrodes, cations and anions
• The products of electrolysis of solutions
• Keyword descriptions and revision tips

GCSE Chemistry: Group 1 - Alkali Metals
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Alkali Metals
• Trends and anomalies in Group 1 (Density, Melting Point)
• Reactivity of Group 1 Alkali Metals
• Electron configuration of Group 1 Alkali Metals

GCSE Chemistry: Group 7 - Halogens
This PowerPoint presentation with worked examples and student questions covers:
• Definition of Alkali Metals
• Properties of Halogens
• Trends and anomalies in Group 7 (Density, Melting Point)
• Reactivity of Group 7 Halogens
• Electron configuration of Group 7 Halogens
• Forming salts with alkali metals and halogens

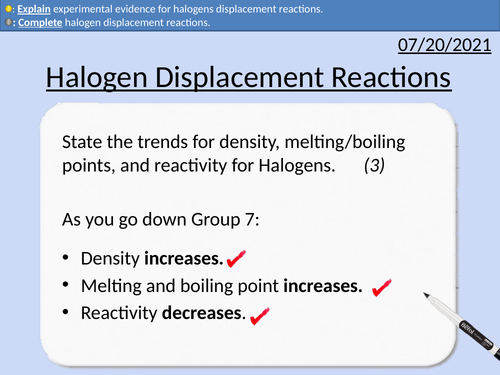
GCSE Chemistry: Halogen Displacement Reactions
This PowerPoint presentation with worked examples and student questions covers:
• Definition of halides displacement reactions
• Definition of displacement reactions
• Identifying displaced products
• Completing displacement reactions
• Explaining experimental evidence for displacement reactions.

GCSE Chemistry: Group 0 - Noble Gases
This PowerPoint presentation with worked examples and student questions covers:
• Properties of Noble gases
• Trends and anomalies in Group 0 (Density, Melting Point)
• Reactivity of Group 0 Noble gases
• Electron configuration of Group 0 Noble gases

GCSE Chemistry: Transition Metals
This PowerPoint presentation with worked examples and student questions covers:
• Properties of transition metals gases
• Comparing transition metals with alkali metals
• Everyday applications of transition metals
• Transition metals as catalysts