497Uploads
167k+Views
71k+Downloads
Chemistry

GCSE Chemistry: Reactivity of Elements
This PowerPoint presentation with worked examples and student questions covers:
• Group 1, 2, 7, 0 electron structures
• Reactivity series for metals
• Equation for metals and water
• Equation for metals and acid
• Displacement reactions for metals

GCSE Chemistry: Detecting Cations
This PowerPoint presentation with worked examples and student questions covers:
Flame tests for lithium, sodium, potassium, calcium, and copper.
Electron energy levels and emitting radiation.
Precipitate tests for iron(II)), iron(III), copper(II), calcium, and zinc.

GCSE Chemistry: Detecting Gases
This PowerPoint presentation with worked examples and student questions covers:
Tests for Hydrogen, Oxygen, Carbon Dioxide, Chlorine.
Gifs of each gas test
Electron structure for diatomic molecules
Bundle

GCSE OCR Chemistry C4.1 Predicting and identifying reactions and products
C4.1 Predicting and identifying reactions and products
All resources for P4.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Group 1 - The Alkali Metals
Group 7 - The Halogens
Halogen Displacement Reactions
Group 0 - The Noble Gases
The Transition Metals
Reactivity of Elements

GCSE Chemistry: Alkenes
This PowerPoint presentation with worked examples and student questions covers:
• Unsaturated hydrocarbons
• Comparing alkanes and alkenes
• Mnemonic device for naming alkenes
• General formula for alkenes
• Completing addition reactions for alkenes

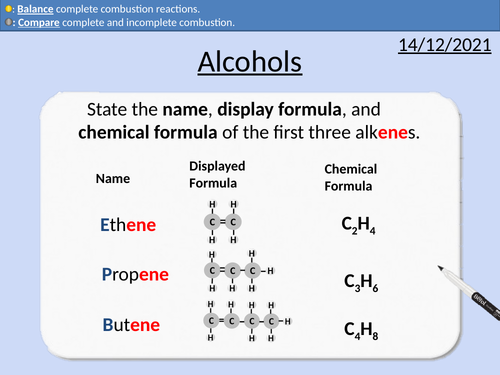
GCSE Chemistry: Alcohols
This PowerPoint presentation with worked examples and student questions covers:
• Functional groups of alcohols, alkanes, and alkenes.
• Comparing incomplete and complete combustion of alcohols
• Mnemonic device for naming alcohols
• General formula for alcohols
• Drawing the structural formula for alcohols

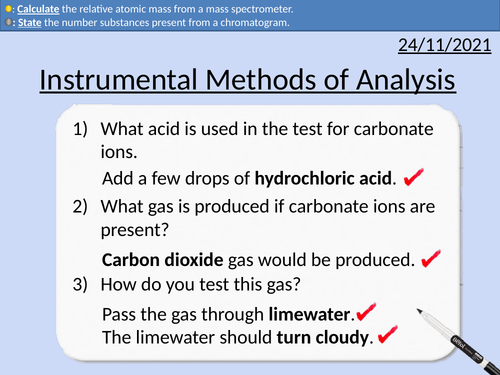
GCSE Chemistry: Instrumental Methods of Analysis
This PowerPoint presentation with worked examples and student questions covers:
Jobs in Environmental Chemistry
Definition of Instrumental Methods of Analysis
Advantages of Instrumental Methods of Analysis
Gas Chromatography and Chromatograms
Mass Spectrometer and Relative Atomic Mass
Identifying a molecule with use of a mass spectrum

GCSE Chemistry: Alkanes
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Carbon and hydrogen saturation
• Mnemonic device for naming alkanes
• Comparing complete and incomplete combustion
• Balancing complete combustion reactions

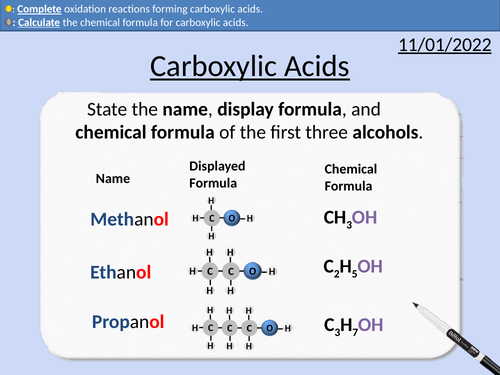
GCSE Chemistry: Carboxylic Acids
This PowerPoint presentation with worked examples and student questions covers:
• Functional groups of carboxylic acids, alcohols, alkanes, and alkenes.
• Mnemonic device for naming carboxylic acids
• General formula for carboxylic acids
• Drawing the structural formula for carboxylic acids
• Carboxylic acids as weak acids and
• Acid reactions with bases, metals, and carbonates
• Oxidation reactions from alcohols to carboxylic acids

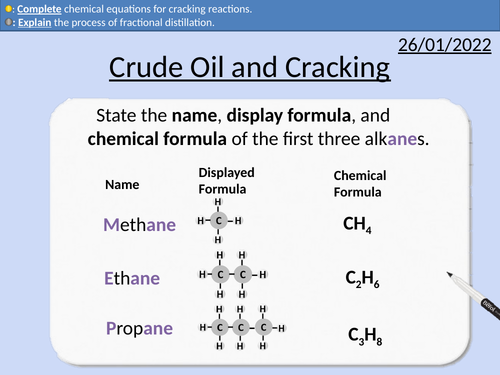
GCSE Chemistry: Crude Oil and Cracking
This PowerPoint presentation with worked examples and student questions covers:
• Definition of hydrocarbons
• Fossil fuels being finite and non-renewable
• Inter-molecular forces and boiling points
• Fractional distillation of crude oil
• Uses of crude oil
• Cracking equations and reasons to crack hydrocarbons

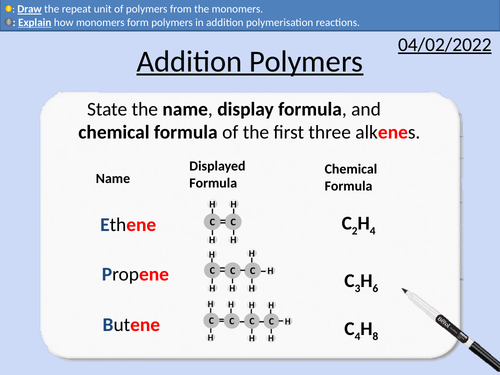
GCSE Chemistry: Addition Polymers
This PowerPoint presentation with worked examples and student questions covers:
Prefixes mono- and poly-
Alkanes and alkenes functional groups
Saturated and unsaturated carbon bonds
Addition polymerisation reactions
Conditions needed for polymerisation reactions
How monomers form polymers
Repeat units and monomers

GCSE Chemistry: Biological Polymers
This PowerPoint presentation with worked examples and student questions covers:
Proteins as polymers and amino acids as monomers
Carbohydrates and simple sugars
Comparing simple sugars (glucose, fructose, and sucrose) with complex carbohydrates (starch).
DNA as a polymer and nucleotides as monomers
Structure of nucleotides (phosphate group,
a sugar (deoxyribose), and an organic base).
Base pairing in DNA and hydrogen bonds

GCSE Chemistry: Condensation Polymers
This PowerPoint presentation with worked examples and student questions covers:
Block notation for hydrocarbons
Amino acids functional groups
Amino acids forming proteins through condensation reactions
Forming polyesters through condensation reactions
Forming polyamides through condensation reactions
Comparing polyesters and polyamides
Conditions for condensation polymers

GCSE Chemistry: Producing Electricity Using Chemistry
This PowerPoint presentation with worked examples and student questions covers:
Chemical cells uses
Fuel cell uses
Comparing fuel cells and chemical cells
Environmental impact of fuel cells and chemical cells
The structure of fuel cells
The operation of fuel cells
Half-equations for fuel cells

OCR AS Chemistry: Nomenclature of Organic Compounds
OCR AS Chemistry: 11.2 Nomenclature of Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Aliphatic, alicyclic, and aromatic compounds.
Naming organic compounds
Drawing organic compounds
Functional Groups
Alkane
Alkene
Alkyne
Alcohols
Haloalkane
Aldehyde
Ketone
Carboxylic Acid
Ester
Amine
Nitrile

OCR AS Chemistry: Organic Chemistry
OCR AS Chemistry: 11.1 Organic Chemistry
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definition of hydrocarbons
What organic chemistry is
Saturated and unsaturated hydrocarbons
Definition of functional groups
Definition of homologous group

OCR AS Chemistry: Representing the formulae of Organic Compounds
OCR AS Chemistry: Formulae for Organic Compounds
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
molecular formula
empirical formula
general formula
displayed formula
structural formula
skeletal formula
Bundle

OCR AS level Chemistry: Basic Concepts of Organic Chemistry
OCR AS level Chemistry: Basic Concepts of Organic Chemistry apart of the Module 4: Core Organic Chemistry and Analysis
All presentations come with worked examples, solutions and homeworks

OCR AS Chemistry: Introduction to Reaction Mechanisms
OCR AS Chemistry: 11.5 Introduction to Reaction Mechanisms
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Covalent bonds
Homolytic fission and heterolytic reactions
Curly arrows in reaction mechanisms
Identifying addition, substitution, and elimination reactions.

OCR AS Chemistry: Structural Isomerism
OCR AS Chemistry: 11.4 Structural Isomerism
This PowerPoint is a whole lessons included with student activities, animated answers, homework questions with answers provided.
This lesson covers:
Definition for Structural Isomers
Moving functional group to form isomers
Aldehydes and ketones being structural isomers
Skeletal formula and structural formula