497Uploads
167k+Views
71k+Downloads
All resources

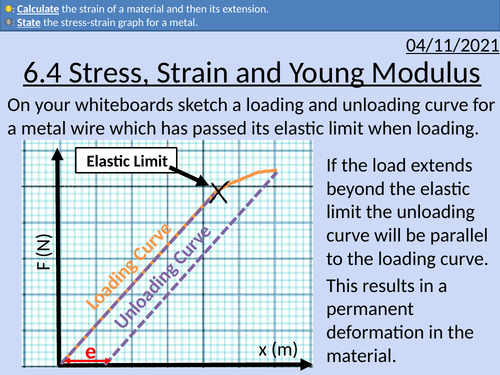
OCR AS level Physics: Stress, Strain, &Young Modulus
OCR AS level Physics: Stress, Strain and Young Modulus is a part of the Module 3: Materials. Presentations come with worked examples, solutions and homeworks.
Bundle

OCR AS level Physics: Materials
OCR AS level Physics presentations for module 3: Materials.
All presentations come with worked examples, solutions and homeworks.
This covers topics from Hooke’s Law to Young Modulus.
Bundle

OCR AS level Physics: Laws of Motion
OCR AS level Physics presentations for module 3: Materials.
All presentations come with worked examples, solutions and homeworks.
This covers topics from Newton’s laws to conservation of momentum in two dimensions.
Bundle

OCR AS level Physics: Work, Energy and Power
OCR AS level Physics presentations for module 3: Work, Energy and Power.
All presentations come with worked examples, solutions and homeworks.
This covers topics from conservation of energy to derivations for kinetic energy.

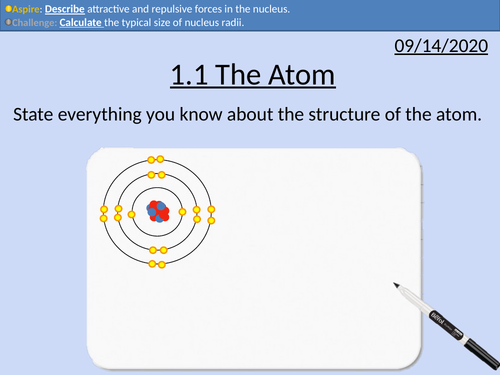
OCR Applied Science: 1.1 The Atom
This PowerPoint presentation with worked examples and student activities covers:
Topic 1.1 of Science Fundementals of the OCR Applied Science Spec.
nucleus contains protons and neutrons surrounded by electrons
relative masses and charges
nuclear and atomic diameters
nucleon number, proton number and isotopes
proton number defines the type of atom
nuclear notation
attractive and repulsive forces within the nucleus

OCR Applied Science: 2.2 Reactions
This PowerPoint presentation with worked examples and student activities covers:
Topic 2.2 of Module 1: Science Fundementals of the OCR Applied Science Spec.
Oxidation and reduction (redox) reactions
Addition reactions of alkenes to include full balanced symbol equations
Substitution reactions of alkanes and haloalkanes to include full balanced
equations
Addition polymerisation to include identification of monomers and repeating units
Condensation polymerisation to include identification of monomers and repeating units
Definition of a radical
The role played by UV light in producing chlorine radicals from CFCs in the
depletion of the ozone layer
Equations to show how chlorine radicals can destroy many ozone molecules
Displacement reactions to include full balanced equations for metals and halogens.

OCR Applied Science: 6.3 Electrical Properties
This PowerPoint presentation with worked examples and student activities covers:
Topic 6.3 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Current as flow of charge in a conductor.
Use the equation: I = ΔQ ÷ Δt
Ohm’s law illustrates the relationship of V ∝ I
Use the equation: potential difference (V) = current (A) × resistance
Use the equations for adding resistors in series and parallel
Compare electromotive force and potential difference
Use the equation: charge © = current (A) × time (s)
Use and recognise the equation for mean drift velocity
Use the equation: energy transferred (work done) (J) = charge © × potential difference (V)
Use the equation: energy transferred (J, kWh) = power (W, kW) × time (s, h)
Use the equation: power (W) = energy (J) ÷ time (s)

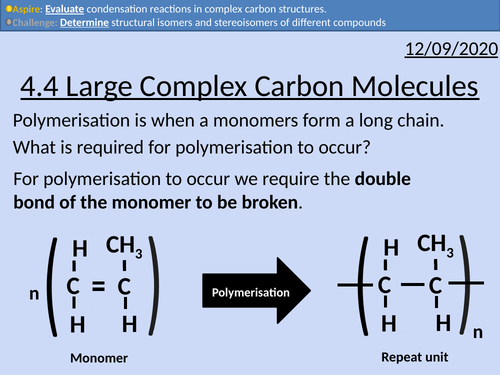
OCR Applied Science: 4.4 Large Complex Carbon Molecules
This PowerPoint presentation with worked examples and student activities covers: Topic 4.4 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
Complex carbohydrates (starch, glycogen, cellulose)
• Carbohydrates found as monosaccharides, disaccharides, or polysaccharides (monomers, dimers or polymers)
• Monomers held together by glycosidic bonds to form dimers and polymers, via condensation reactions
• Monosaccharides include glucose, fructose and galactose
• Disaccharides include maltose, sucrose and lactose
• Polysaccharides include starch, glycogen and cellulose
• Cellulose is found in plant cell walls where it provides strength/support and pliability
• Starch and glycogen are energy sources
Proteins and peptides from amino acids
• Dipeptides are formed from two amino acids joined by a peptide bond, via a condensation reaction
• Polypeptides are chains of amino acids joined by peptide bonds
• Proteins/polypeptides have physiological or functional roles, including enzymes, carrier proteins in the plasma membrane, and structural roles, including collagen and elastin fibres in connective tissue
Lipids from fatty acids, glycerol and phosphorus compounds
• Monoglycerides, diglycerides and triglycerides are esters of fatty acids and glycerol
• An ester bond forms between each fatty acid and the glycerol, via condensation reactions
• Phospholipids contain glycerol plus two fatty acids and a phosphate group
• Lipids act as an energy source within cells, as an insulation layer around animal organs, in the myelin sheath (found around some nerve fibres/axons) to increase speed of nerve transmission
• Phospholipids form a bilayer in the plasma membrane
Protein synthesis (transcription, translation) RNA, messenger, ribosomal and transfer
• The nucleic acids, DNA and RNA, are polymers of nucleotides
• Peptide bonds form between amino acids to create polypeptide chains/proteins
• Recall a simple description of protein synthesis

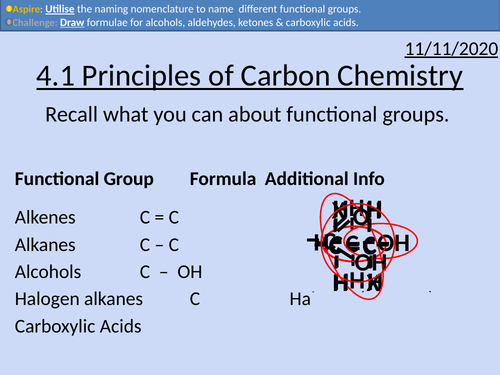
OCR Applied Science: 4.1 Principles of Carbon Chemistry
This PowerPoint presentation with worked examples and student activities covers:
Topic 4.1 of Module 1: Science Fundamentals of the OCR Applied Science Spec.
• Alkanes as saturated hydrocarbons containing single C-C and C-H bonds
• Alkenes as unsaturated hydrocarbons containing a C=C double bond
• Alkynes as unsaturated hydrocarbons containing a C ≡ C triple bond
• Name and draw structural and skeletal formulae of the first four members of alkanes, alkenes and alkynes
• Aldehydes and ketones as organic compounds containing the C=O group
• Name and draw the structural formulae of the first four aldehydes and the first two ketones
• Alcohols as organic compounds containing the OH group
• Name and draw structural and skeletal formulae of the first four alcohols
• Conversion of alcohols to form aldehydes and ketones is classified as an oxidation reaction
• Name and draw structural and skeletal formulae of the first four carboxylic acids
• Reaction of carboxylic acids with an alkali, to include full equations using structural formulae
• Name and draw structural and skeletal formulae of the four C4H8O2 esters
• How an ester can be made from a carboxylic acid and an alcohol
Bundle

GCSE OCR Chemistry C2.1 Purity and Separating Mixtures
All resources for P2.1 GCSE OCR Chemistry Gateway 9-1 Triple and combined (Higher and Foundation) is covered in this material.
Includes:
Relative Formula Mass
Empirical Formula
Pure and Impure Substances
Filtration and Crystallisation
Simple Distillation
Paper Chromatography
Gas and Think Layer Chromatography
Purification and Checking Purity

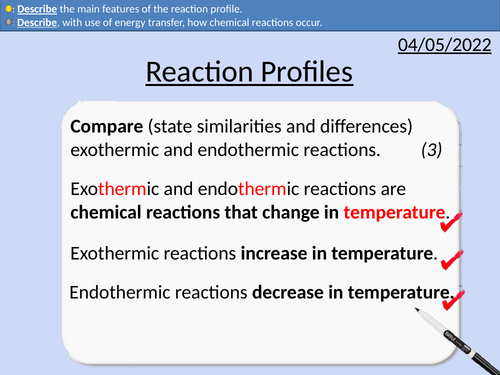
GCSE Chemistry: Reaction Profiles
This PowerPoint presentation with worked examples and student questions covers:
• Reaction profiles for exothermic and endothermic
• Energy stores of particles and surroundings
• Activation energy
• Describing the main features of reaction profiles.

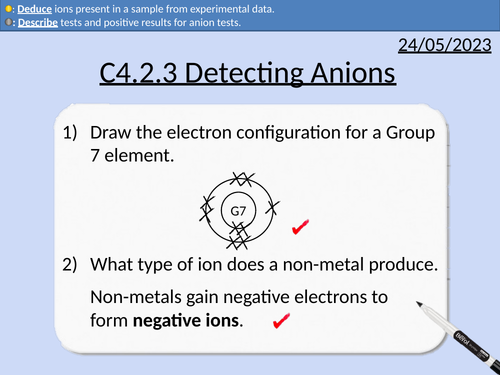
GCSE Chemistry: Detecting Anions
This PowerPoint presentation with worked examples and student questions covers:
Definitions for anions, cations, anodes, cathodes.
Tests for carbonate ions
Tests for sulfate ions
Tests for halide ions

OCR AS Physics: Analysing Circuits
OCR AS Physics: Analysing Circuits is a part of the Module 4: Electrons, Waves, and Photons. PowerPoint with worked examples and homework.
Drawing and labeling circuit diagrams
Adding Resistors in Series and Parallel
Ohm’s Law
Kirchhoff’s Laws
The Electrical Power Equation

Scientist of the week reward system
A simple scientist of the week reward system display is included which highlights women and PoC in STEM jobs.

OCR A level Physics: Solids, Liquids and Gases
OCR A level Physics: Solids, Liquids and Gases is a part of the Module 5: Newtonian World and Astrophysics. The PowerPoint presentation includes worked examples, solutions and a homework.

GCSE Physics: Absorption and Emission Spectra
This presentation covers OCR Gateway Physics 9-1 P6.1.5 Radiation in and out of atoms.
This PowerPoint is a whole lessons included with student activities and animated answers.
Arrangements of electrons and distance from the nucleus
Electron energy levels
Absorbing electromagnetic radiation
Electromagnetic radiation and energy
Absorption spectra
Emission spectra
Discovery of helium

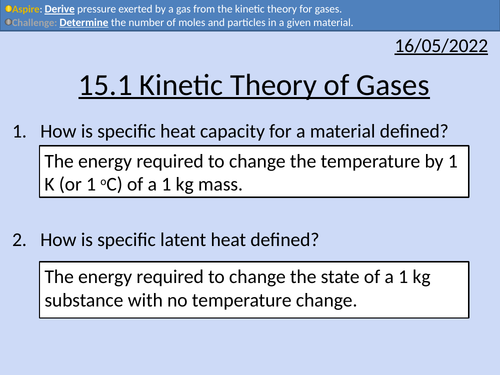
OCR A level Physics: Kinetic Theory of Gases
OCR A level Physics: Kinetic Theory of Gases is a part of the Module 5: Newtonian World and Astrophysics. The PowerPoint presentation includes worked examples, solutions and a homework.

OCR A level Physics: Specific Latent Heat
OCR A level Physics: Specific Latent Heat is a part of the Module 5: Newtonian World and Astrophysics. The PowerPoint presentation includes worked examples, solutions and a homework.

OCR A level Physics: Internal Energy
OCR A level Physics: Internal Energy is a part of the Module 5: Newtonian World and Astrophysics. The PowerPoint presentation includes worked examples, solutions and a homework.

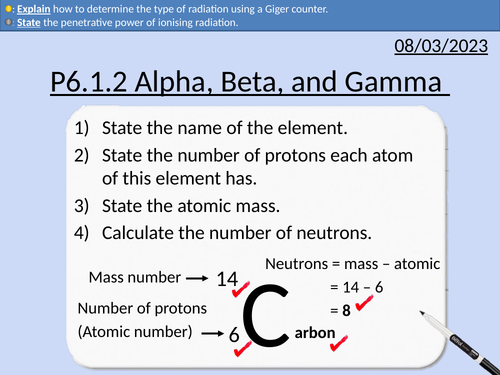
GCSE Physics: Alpha, Beta, Gamma Radiation
This presentation covers OCR Gateway Physics 9-1 P6.1.2 Alpha, Beta, Gamma Radiation.
All presentations come with student activities and worked solutions.
Types of radiation - alpha, beta, gamma, and neutrons
Ions and Atoms
Ionising Radiation
Geiger counter
Range and penetrative power
Precautions using radiation sources
Exam question with solution